Proteopathy
In medicine, proteopathy (/proʊtiːˈɒpəθiː/; from proteo- [pref. protein]; -pathy [suff. disease]; proteopathies pl.; proteopathic adj) refers to a class of diseases in which certain proteins become structurally abnormal, and thereby disrupt the function of cells, tissues and organs of the body. Often the proteins fail to fold into their normal configuration; in this misfolded state, the proteins can become toxic in some way (a toxic gain-of-function) or they can lose their normal function. The proteopathies (also known as proteinopathies, protein conformational disorders, or protein misfolding diseases) include such diseases as Creutzfeldt–Jakob disease and other prion diseases, Alzheimer's disease, Parkinson's disease, amyloidosis, multiple system atrophy, and a wide range of other disorders. The term proteopathy was first proposed in 2000 by Lary Walker and Harry LeVine.
The concept of proteopathy can trace its origins to the mid-19th century, when, in 1854, Rudolf Virchow coined the term amyloid ("starch-like") to describe a substance in cerebral corpora amylacea that exhibited a chemical reaction resembling that of cellulose. In 1859, Friedreich and Kekulé demonstrated that, rather than consisting of cellulose, "amyloid" actually is rich in protein. Subsequent research has shown that many different proteins can form amyloid, and that all amyloids show birefringence in cross-polarized light after staining with the dye Congo red, as well as a fibrillar ultrastructure when viewed with an electron microscope. However, some proteinaceous lesions lack birefringence and contain few or no classical amyloid fibrils, such as the diffuse deposits of amyloid beta (Aβ) protein in the brains of people with Alzheimer's. Furthermore, evidence has emerged that small, non-fibrillar protein aggregates known as oligomers are toxic to the cells of an affected organ, and that amyloidogenic proteins in their fibrillar form may be relatively benign.
Pathophysiology
In most, if not all proteopathies, a change in 3-dimensional folding (conformation) increases the tendency of a specific protein to bind to itself. In this aggregated form, the protein is resistant to clearance and can interfere with the normal capacity of the affected organs. In some cases, misfolding of the protein results in a loss of its usual function. For example, cystic fibrosis is caused by a defective cystic fibrosis transmembrane conductance regulator (CFTR) protein, and in amyotrophic lateral sclerosis / frontotemporal lobar degeneration (FTLD), certain gene-regulating proteins inappropriately aggregate in the cytoplasm, and thus are unable to perform their normal tasks within the nucleus. Because proteins share a common structural feature known as the polypeptide backbone, all proteins have the potential to misfold under some circumstances. However, only a relatively small number of proteins are linked to proteopathic disorders, possibly due to structural idiosyncrasies of the vulnerable proteins. For example, proteins that are normally unfolded or relatively unstable as monomers (that is, as single, unbound protein molecules) are more likely to misfold into an abnormal conformation. In nearly all instances, the disease-causing molecular configuration involves an increase in beta-sheet secondary structure of the protein. The abnormal proteins in some proteopathies have been shown to fold into multiple 3-dimensional shapes; these variant, proteinaceous structures are defined by their different pathogenic, biochemical, and conformational properties. They have been most thoroughly studied with regard to prion disease, and are referred to as protein strains.

The likelihood that proteopathy will develop is increased by certain risk factors that promote the self-assembly of a protein. These include destabilizing changes in the primary amino acid sequence of the protein, post-translational modifications (such as hyperphosphorylation), changes in temperature or pH, an increase in production of a protein, or a decrease in its clearance. Advancing age is a strong risk factor, as is traumatic brain injury. In the aging brain, multiple proteopathies can overlap. For example, in addition to tauopathy and Aβ-amyloidosis (which coexist as key pathologic features of Alzheimer's disease), many Alzheimer patients have concomitant synucleinopathy (Lewy bodies) in the brain.
It is hypothesized that chaperones and co-chaperones (proteins that assist protein folding) may antagonize proteotoxicity during aging and in protein misfolding-diseases to maintain proteostasis.
Seeded induction
Some proteins can be induced to form abnormal assemblies by exposure to the same (or similar) protein that has folded into a disease-causing conformation, a process called 'seeding' or 'permissive templating'. In this way, the disease state can be brought about in a susceptible host by the introduction of diseased tissue extract from an afflicted donor. The best known form of such inducible proteopathy is prion disease, which can be transmitted by exposure of a host organism to purified prion protein in a disease-causing conformation. There is now evidence that other proteopathies can be induced by a similar mechanism, including Aβ amyloidosis, amyloid A (AA) amyloidosis, and apolipoprotein AII amyloidosis, tauopathy, synucleinopathy, and the aggregation of superoxide dismutase-1 (SOD1), polyglutamine, and TAR DNA-binding protein-43 (TDP-43).
In all of these instances, an aberrant form of the protein itself appears to be the pathogenic agent. In some cases, the deposition of one type of protein can be experimentally induced by aggregated assemblies of other proteins that are rich in β-sheet structure, possibly because of structural complementarity of the protein molecules. For example, AA amyloidosis can be stimulated in mice by such diverse macromolecules as silk, the yeast amyloid Sup35, and curli fibrils from the bacterium Escherichia coli. AII amyloid can be induced in mice by a variety of β-sheet rich amyloid fibrils, and cerebral tauopathy can be induced by brain extracts that are rich in aggregated Aβ. There is also experimental evidence for cross-seeding between prion protein and Aβ. In general, such heterologous seeding is less efficient than is seeding by a corrupted form of the same protein.
List of proteopathies
| Proteopathy | Major aggregating protein |
| Alzheimer's disease | Amyloid β peptide (Aβ); Tau protein (see tauopathies) |
| Cerebral β-amyloid angiopathy | Amyloid β peptide (Aβ) |
| Retinal ganglion cell degeneration in glaucoma | Amyloid β peptide (Aβ) |
| Prion diseases (multiple) | Prion protein |
| Parkinson's disease and other synucleinopathies (multiple) | α-Synuclein |
| Tauopathies (multiple) | Microtubule-associated protein tau (Tau protein) |
| Frontotemporal lobar degeneration (FTLD) (Ubi+, Tau-) | TDP-43 |
| FTLD–FUS | Fused in sarcoma (FUS) protein |
| Amyotrophic lateral sclerosis (ALS) | Superoxide dismutase, TDP-43, FUS, C9ORF72, ubiquilin-2 (UBQLN2) |
| Huntington's disease and other trinucleotide repeat disorders (multiple) | Proteins with tandem glutamine expansions |
| Familial British dementia | ABri |
| Familial Danish dementia | ADan |
| Hereditary cerebral hemorrhage with amyloidosis (Icelandic) (HCHWA-I) | Cystatin C |
| CADASIL | Notch3 |
| Alexander disease | Glial fibrillary acidic protein (GFAP) |
| Pelizaeus-Merzbacher disease | proteolipid protein (PLP) |
| Seipinopathies | Seipin |
| Familial amyloidotic neuropathy, Senile systemic amyloidosis | Transthyretin |
| Serpinopathies (multiple) | Serpins |
| AL (light chain) amyloidosis (primary systemic amyloidosis) | Monoclonal immunoglobulin light chains |
| AH (heavy chain) amyloidosis | Immunoglobulin heavy chains |
| AA (secondary) amyloidosis | Amyloid A protein |
| Type II diabetes | Islet amyloid polypeptide (IAPP; amylin) |
| Aortic medial amyloidosis | Medin (lactadherin) |
| ApoAI amyloidosis | Apolipoprotein AI |
| ApoAII amyloidosis | Apolipoprotein AII |
| ApoAIV amyloidosis | Apolipoprotein AIV |
| Familial amyloidosis of the Finnish type (FAF) | Gelsolin |
| Lysozyme amyloidosis | Lysozyme |
| Fibrinogen amyloidosis | Fibrinogen |
| Dialysis amyloidosis | Beta-2 microglobulin |
| Inclusion body myositis/myopathy | Amyloid β peptide (Aβ) |
| Cataracts | Crystallins |
| Retinitis pigmentosa with rhodopsin mutations | rhodopsin |
| Medullary thyroid carcinoma | Calcitonin |
| Cardiac atrial amyloidosis | Atrial natriuretic factor |
| Pituitary prolactinoma | Prolactin |
| Hereditary lattice corneal dystrophy | Keratoepithelin |
| Cutaneous lichen amyloidosis | Keratins |
| Mallory bodies | Keratin intermediate filament proteins |
| Corneal lactoferrin amyloidosis | Lactoferrin |
| Pulmonary alveolar proteinosis | Surfactant protein C (SP-C) |
| Odontogenic (Pindborg) tumor amyloid | Odontogenic ameloblast-associated protein |
| Seminal vesicle amyloid | Semenogelin I |
| Apolipoprotein C2 amyloidosis | Apolipoprotein C2 (ApoC2) |
| Apolipoprotein C3 amyloidosis | Apolipoprotein C3 (ApoC3) |
| Lect2 amyloidosis | Leukocyte chemotactic factor-2 (Lect2) |
| Insulin amyloidosis | Insulin |
| Galectin-7 amyloidosis (primary localized cutaneous amyloidosis) | Galectin-7 (Gal7) |
| Corneodesmosin amyloidosis | Corneodesmosin |
| Enfuvirtide amyloidosis | Enfuvirtide |
| Cystic fibrosis | cystic fibrosis transmembrane conductance regulator (CFTR) protein |
| Sickle cell disease | Hemoglobin |
Management
The development of effective treatments for many proteopathies has been challenging. Because the proteopathies often involve different proteins arising from different sources, treatment strategies must be customized to each disorder; however, general therapeutic approaches include maintaining the function of affected organs, reducing the formation of the disease-causing proteins, preventing the proteins from misfolding and/or aggregating, or promoting their removal. For example, in Alzheimer's disease, researchers are seeking ways to reduce the production of the disease-associated protein Aβ by inhibiting the enzymes that free it from its parent protein. Another strategy is to use antibodies to neutralize specific proteins by active or passive immunization. In some proteopathies, inhibiting the toxic effects of protein oligomers might be beneficial. Amyloid A (AA) amyloidosis can be reduced by treating the inflammatory state that increases the amount of the protein in the blood (referred to as serum amyloid A, or SAA). In immunoglobulin light chain amyloidosis (AL amyloidosis), chemotherapy can be used to lower the number of the blood cells that make the light chain protein that forms amyloid in various bodily organs. Transthyretin (TTR) amyloidosis (ATTR) results from the deposition of misfolded TTR in multiple organs. Because TTR is mainly produced in the liver, TTR amyloidosis can be slowed in some hereditary cases by liver transplantation. TTR amyloidosis also can be treated by stabilizing the normal assemblies of the protein (called tetramers because they consist of four TTR molecules bound together). Stabilization prevents individual TTR molecules from escaping, misfolding, and aggregating into amyloid.
Several other treatment strategies for proteopathies are being investigated, including small molecules and biologic medicines such as small interfering RNAs, antisense oligonucleotides, peptides, and engineered immune cells. In some cases, multiple therapeutic agents may be combined to improve effectiveness.
Additional images

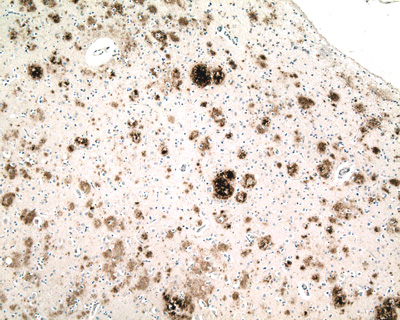
Micrograph of tauopathy (brown) in a neuronal cell body (arrow) and process (arrowhead) in the cerebral cortex of a patient with Alzheimer's disease. Bar = 25 microns (0.025mm).
See also
- Amyloidosis
- Neurofibrillary tangles
- Protein toxicity
- Prion
