Coronavirus Disease 2019

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case was identified in Wuhan, China, in December 2019. It has since spread worldwide, leading to an ongoing pandemic.
Symptoms of COVID-19 are variable, but often include fever, cough, fatigue, breathing difficulties, and loss of smell and taste. Symptoms begin one to fourteen days after exposure to the virus. Around one in five infected individuals do not develop any symptoms. While most people have mild symptoms, some people develop acute respiratory distress syndrome (ARDS). ARDS can be precipitated by cytokine storms, multi-organ failure, septic shock, and blood clots. Longer-term damage to organs (in particular, the lungs and heart) has been observed. There is concern about a significant number of patients who have recovered from the acute phase of the disease but continue to experience a range of effects—known as long COVID—for months afterwards. These effects include severe fatigue, memory loss and other cognitive issues, low-grade fever, muscle weakness, and breathlessness.
The virus that causes COVID-19 spreads mainly when an infected person is in close contact with another person. Small droplets and aerosols containing the virus can spread from an infected person's nose and mouth as they breathe, cough, sneeze, sing, or speak. Other people are infected if the virus gets into their mouth, nose or eyes. The virus may also spread via contaminated surfaces, although this is not thought to be the main route of transmission. The exact route of transmission is rarely proven conclusively, but infection mainly happens when people are near each other for long enough. It can spread as early as two days before infected persons show symptoms, and from individuals who never experience symptoms. People remain infectious for up to ten days in moderate cases, and two weeks in severe cases. Various testing methods have been developed to diagnose the disease. The standard diagnosis method is by real-time reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab.
Preventive measures include physical or social distancing, quarantining, ventilation of indoor spaces, covering coughs and sneezes, hand washing, and keeping unwashed hands away from the face. The use of face masks or coverings has been recommended in public settings to minimise the risk of transmissions. Several vaccines have been developed and various countries have initiated mass vaccination campaigns.
Although work is underway to develop drugs that inhibit the virus, the primary treatment is currently symptomatic. Management involves the treatment of symptoms, supportive care, isolation, and experimental measures.
Signs and symptoms

Symptoms of COVID-19 are variable, ranging from mild symptoms to severe illness. Common symptoms include headache, loss of smell and taste, nasal congestion and rhinorrhea, cough, muscle pain, sore throat, fever and breathing difficulties. People with the same infection may have different symptoms, and their symptoms may change over time. In people without prior ears, nose, and throat disorders, loss of taste combined with loss of smell is associated with COVID-19 with a specificity of 95%.
Most people (81%) develop mild to moderate symptoms (up to mild pneumonia), while 14% develop severe symptoms (dyspnea, hypoxia, or more than 50% lung involvement on imaging) and 5% of patients suffer critical symptoms (respiratory failure, shock, or multiorgan dysfunction). Around one in five people are infected with the virus but do not develop noticeable symptoms at any point in time. A June 2020 review asserted that asymptomatic infections might be as high as 40 to 45 percent with the ability to transmit the virus for a period that extends beyond two weeks. These asymptomatic carriers tend not to get tested, and they can spread the disease. Other infected people will develop symptoms later (called pre-symptomatic) or have very mild symptoms, and can also spread the virus.
As is common with infections, there is a delay, known as the incubation period, between the moment a person first becomes infected and the appearance of the first symptoms. The median incubation period for COVID-19 is four to five days. Most symptomatic people experience symptoms within two to seven days after exposure, and almost all symptomatic people will experience one or more symptoms before day twelve.Cause
COVID-19 is caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus strain.
Transmission
COVID-19 spreads from person to person mainly through the respiratory route after an infected person coughs, sneezes, sings, talks or breathes. A new infection occurs when virus-containing particles exhaled by an infected person, either respiratory droplets or aerosols, get into the mouth, nose, or eyes of other people who are in close contact with the infected person. During human-to-human transmission, an average 1000 infectious SARS-CoV-2 virions are thought to initiate a new infection.
The closer people interact, and the longer they interact, the more likely they are to transmit COVID-19. Closer distances can involve larger droplets (which fall to the ground) and aerosols, whereas longer distances only involve aerosols. The larger droplets may also evaporate into the aerosols (known as droplet nuclei). The relative importance of the larger droplets and the aerosols is not clear as of November 2020, however the virus is not known to transmit between rooms over long distances such as through air ducts. Airborne transmission is able to particularly occur indoors, in high risk locations, such as in restaurants, choirs, gyms, nightclubs, offices, and religious venues, often when they are crowded or less ventilated. It also occurs in healthcare settings, often when aerosol-generating medical procedures are performed on COVID-19 patients.
Social distancing and the wearing of cloth face masks, surgical masks, respirators, or other face coverings are controls for droplet transmission. Transmission may be decreased indoors with well maintained heating and ventilation systems to maintain good air circulation and increase the use of outdoor air.
The number of people generally infected by one infected person varies; as of September 2020 it was estimated that one infected person will, on average, infect between two and three other people. This is more infectious than influenza, but less so than measles. It often spreads in clusters, where infections can be traced back to an index case or geographical location. There is a major role of "super-spreading events", where many people are infected by one person.Virology

Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus. It was first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan. All features of the novel SARS-CoV-2 virus occur in related coronaviruses in nature.
Outside the human body, the virus is destroyed by household soap, which bursts its protective bubble.
SARS-CoV-2 is closely related to the original SARS-CoV. It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13). The structural proteins of SARS-CoV-2 include membrane glycoprotein (M), envelope protein (E), nucleocapsid protein (N), and the spike protein (S). The M protein of SARS-CoV-2 is 98.6% similar to the M protein of bat SARS-CoV, maintains 98.2% homology with pangolin SARS-CoV, and has 90% homology with the M protein of SARS-CoV; whereas, the similarity is only 38% with the M protein of MERS-CoV. In silico analyses showed that the M protein of SARS-CoV-2 has a triple helix bundle, forms a single 3-transmembrane domain, and is homologous to the prokaryotic sugar transport protein SemiSWEET.
The many thousands SARS-CoV-2 variants are grouped into clades. Several different clade nomenclatures have been proposed. Nextstrain divides the variants into five clades (19A, 19B, 20A, 20B, and 20C), while GISAID divdes them into seven (L, O, V, S, G, GH, and GR).
Several notable variants of SARS-CoV-2 emerged in the fall of 2020. Cluster 5 emerged among minks and mink farmers in Denmark. After strict quarantines and a mink euthanasia campaign, it is believed to have been eradicated. The Variant of Concern 202012/01 (VOC 202012/01) is believed to have emerged in the United Kingdom in September. The 501Y.V2 Variant, which has the same N501Y mutation, arose independently in South Africa.
Pathophysiology
COVID-19 can affect the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs). The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant in type II alveolar cells of the lungs. The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to ACE2 and enter the host cell. The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested decreasing ACE2 activity might be protective, though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective. As the alveolar disease progresses, respiratory failure might develop and death may follow.
Whether SARS-CoV-2 is able to invade the nervous system remains unknown. The virus is not detected in the CNS of the majority of COVID-19 patients with neurological issues. However, SARS-CoV-2 has been detected at low levels in the brains of patients who died from COVID-19, but these results need to be confirmed. SARS-CoV-2 may cause respiratory failure through affecting the brain stem as other coronaviruses have been found to invade the CNS. While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain. The virus may also enter the bloodstream from the lungs and cross the blood-brain barrier to gain access to the CNS, possibly within an infected white blood cell by a "Trojan horse" mechanism.
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium as well as endothelial cells and enterocytes of the small intestine.
The virus can cause acute myocardial injury and chronic damage to the cardiovascular system. An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China, and is more frequent in severe disease. Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart. ACE2 receptors are highly expressed in the heart and are involved in heart function. A high incidence of thrombosis and venous thromboembolism have been found in intensive care unit (ICU)-transferred patients with COVID-19 infections, and may be related to poor prognosis. Blood vessel dysfunction and clot formation (as suggested by high D-dimer levels) are thought to play a significant role in mortality, incidences of clots leading to pulmonary embolisms, and ischaemic events within the brain have been noted as complications leading to death in patients infected with SARS-CoV-2. Infection appears to set off a chain of vasoconstrictive responses within the body, constriction of blood vessels within the pulmonary circulation has also been posited as a mechanism in which oxygenation decreases alongside the presentation of viral pneumonia.
Another common cause of death is complications related to the kidneys. Early reports show that up to 30% of hospitalized patients both in China and in New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.
Autopsies of people who died of COVID-19 have found diffuse alveolar damage (DAD), and lymphocyte-containing inflammatory infiltrates within the lung.
Immunopathology
Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, patients with severe COVID-19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), Macrophage inflammatory protein 1-α (MIP-1α), and tumour necrosis factor-α (TNF-α) indicative of cytokine release syndrome (CRS) suggest an underlying immunopathology.
Additionally, people with COVID-19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T-cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in COVID-19 patients. Lymphocytic infiltrates have also been reported at autopsy.
Diagnosis


COVID-19 can provisionally be diagnosed on the basis of symptoms and confirmed using reverse transcription polymerase chain reaction (RT-PCR) testing of infected secretions. Along with laboratory testing, chest CT scans may be helpful to diagnose COVID-19 in individuals with a high clinical suspicion of infection. Detection of prior infection is possible with serological tests, which detect antibodies produced by the body in response to infection.
Viral testing
The standard method of testing for presence of SARS-CoV-2 is real-time reverse transcription polymerase chain reaction (rRT-PCR), which detects the presence of viral RNA fragments. As this test detects RNA but not infectious virus, its "ability to determine duration of infectivity of patients is limited." The test is typically done on respiratory samples obtained by a nasopharyngeal swab; however, a nasal swab or sputum sample may also be used. Results are generally available within a few hours to two days. Blood tests can be used, but these require two blood samples taken two weeks apart, and the results have little immediate value. The WHO has published several testing protocols for the disease.
A number of laboratories and companies have developed serological tests, which detect antibodies produced by the body in response to infection. Several have been evaluated by Public Health England and approved for use in the UK.
On 22 June 2020, UK health secretary Matt Hancock announced the country would conduct a new "spit test" for COVID-19 on 14,000 key workers and their families in Southampton, having them spit in a pot, which was collected by Southampton University, with results expected within 48 hours. Hancock said the test was easier than using swabs and could enable people to conduct it at home.
The University of Oxford's CEBM has pointed to mounting evidence that "a good proportion of 'new' mild cases and people re-testing positives after quarantine or discharge from hospital are not infectious, but are simply clearing harmless virus particles which their immune system has efficiently dealt with" and have called for "an international effort to standardize and periodically calibrate testing" On 7 September, the UK government issued "guidance for procedures to be implemented in laboratories to provide assurance of positive SARS-CoV-2 RNA results during periods of low prevalence, when there is a reduction in the predictive value of positive test results."
Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus. As of 4 April 2020[update], antibody tests (which may detect active infections and whether a person had been infected in the past) were in development, but not yet widely used. Antibody tests may be most accurate 2–3 weeks after a person's symptoms start. The Chinese experience with testing has shown the accuracy is only 60 to 70%. The US Food and Drug Administration (FDA) approved the first point-of-care test on 21 March 2020 for use at the end of that month. The absence or presence of COVID-19 signs and symptoms alone is not reliable enough for an accurate diagnosis. Different clinical scores were created based on symptoms, laboratory parameters and imaging to determine patients with probable SARS-CoV-2 infection or more severe stages of COVID-19.
A study asked hospitalised COVID-19 patients to cough into a sterile container, thus producing a saliva sample, and detected the virus in eleven of twelve patients using RT-PCR. This technique has the potential of being quicker than a swab and involving less risk to health care workers (collection at home or in the car).
In November 2020, research showed that breath analysis could make the "rapid identification" in seconds for coronavirus possible.
Imaging
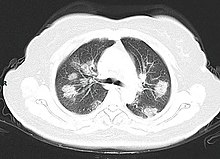
Chest CT scans may be helpful to diagnose COVID-19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening. Bilateral multilobar ground-glass opacities with a peripheral, asymmetric, and posterior distribution are common in early infection. Subpleural dominance, crazy paving (lobular septal thickening with variable alveolar filling), and consolidation may appear as the disease progresses. Characteristic imaging features on chest radiographs and computed tomography (CT) of people who are symptomatic include asymmetric peripheral ground-glass opacities without pleural effusions.
Many groups have created COVID-19 datasets that include imagery such as the Italian Radiological Society which has compiled an international online database of imaging findings for confirmed cases. Due to overlap with other infections such as adenovirus, imaging without confirmation by rRT-PCR is of limited specificity in identifying COVID-19. A large study in China compared chest CT results to PCR and demonstrated that though imaging is less specific for the infection, it is faster and more sensitive.

CT scan of rapid progression stage of COVID-19.

Chest X-ray showing COVID-19 pneumonia.
Coding
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID-19 without lab-confirmed SARS-CoV-2 infection.
Pathology
The main pathological findings at autopsy are:
- Macroscopy: pericarditis, lung consolidation and pulmonary oedema
- Lung findings:
- minor serous exudation, minor fibrin exudation
- pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation
- diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxemia.
- organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis
- plasmocytosis in BAL
- Blood: disseminated intravascular coagulation (DIC); leukoerythroblastic reaction
- Liver: microvesicular steatosis
Prevention


Preventive measures to reduce the chances of infection include staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, ventilating indoor spaces, washing hands with soap and water often and for at least 20 seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands. Those diagnosed with COVID-19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.
The first COVID-19 vaccine was granted regulatory approval on 2 December by the UK medicines regulator MHRA. It was evaluated for emergency use authorization (EUA) status by the US FDA, and in several other countries. Initially, the US National Institutes of Health guidelines do not recommend any medication for prevention of COVID-19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial. Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID-19 is trying to decrease and delay the epidemic peak, known as "flattening the curve". This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of current cases, and delaying additional cases until effective treatments or a vaccine become available.
Vaccine


A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against COVID‑19. Prior to the COVID‑19 pandemic, work to develop a vaccine against the coronavirus diseases SARS and MERS had established knowledge about the structure and function of coronaviruses, which accelerated development during early 2020 of varied technology platforms for a COVID‑19 vaccine.
By mid-December 2020, 57 vaccine candidates were in clinical research, including 40 in Phase I–II trials and 17 in Phase II–III trials. In Phase III trials, several COVID‑19 vaccines demonstrated efficacy as high as 95% in preventing symptomatic COVID‑19 infections. As of January 2021, nine vaccines have been authorized by at least one national regulatory authority for public use: two RNA vaccines (the Pfizer-BioNTech vaccine and the Moderna vaccine), three conventional inactivated vaccines (BBIBP-CorV from Sinopharm, BBV152 from Bharat Biotech and CoronaVac from Sinovac), three viral vector vaccines (Gam-COVID-Vac from the Gamaleya Research Institute, the Oxford–AstraZeneca vaccine, and Sputnik V), and one peptide vaccine (EpiVacCorona).
Many countries have implemented phased distribution plans that prioritize those at highest risk of complications, such as the elderly, and those at high risk of exposure and transmission, such as healthcare workers. As of 14 January 2021, 32.64 million doses of COVID‑19 vaccine had been administered worldwide based on official reports from national health agencies. Pfizer, Moderna, and AstraZeneca predicted a manufacturing capacity of 5.3 billion doses in 2021, which could be used to vaccinate about 3 billion people (as the vaccines require two doses for a protective effect against COVID‑19). By December, more than 10 billion vaccine doses had been preordered by countries, with about half of the doses purchased by high-income countries comprising only 14% of the world's population.Social distancing
Social distancing (also known as physical distancing) includes infection control actions intended to slow the spread of the disease by minimising close contact between individuals. Methods include quarantines; travel restrictions; and the closing of schools, workplaces, stadiums, theatres, or shopping centres. Individuals may apply social distancing methods by staying at home, limiting travel, avoiding crowded areas, using no-contact greetings, and physically distancing themselves from others. Many governments are now mandating or recommending social distancing in regions affected by the outbreak. Non-cooperation with distancing measures in some areas has contributed to the further spread of the pandemic. Initial recommendations included maintaining a six-foot/two-meter distance from others outside the family unit. However, a case occurring in South Korea suggested that is inadequate, citing transmission despite a brief exposure (5 minutes) at 20 feet from the carrier in a restaurant. The maximum gathering size recommended by U.S. government bodies and health organizations was swiftly reduced from 250 people (if there were no known COVID-19 spread in a region) to 50 people, and later to 10. A Cochrane review found that early quarantine with other public health measures are effective in limiting the pandemic. The best manner of adopting and relaxing policies are uncertain, however, as local conditions vary.
Older adults and those with underlying medical conditions such as diabetes, heart disease, respiratory disease, hypertension, and compromised immune systems face increased risk of serious illness and complications and have been advised by the CDC to stay home as much as possible in areas of community outbreak.
In late March 2020, the WHO and other health bodies began to replace the use of the term "social distancing" with "physical distancing", to clarify that the aim is to reduce physical contact while maintaining social connections, either virtually or at a distance. The use of the term "social distancing" had led to implications that people should engage in total social isolation, rather than encouraging them to stay in contact through alternative means. Some authorities have issued sexual health guidelines for responding to the pandemic, which include recommendations to have sex only with someone with whom you live, and who does not have the virus or symptoms of the virus.
Outbreaks have occurred in prisons due to crowding and an inability to enforce adequate social distancing. In the United States, the prisoner population is aging and many of them are at high risk for poor outcomes from COVID-19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.
Self-isolation
Self-isolation at home has been recommended for those diagnosed with COVID-19 and those who suspect they have been infected. Health agencies have issued detailed instructions for proper self-isolation.
Many governments have mandated or recommended self-quarantine for entire populations. The strongest self-quarantine instructions have been issued to those in high-risk groups. Those who may have been exposed to someone with COVID-19 and those who have recently travelled to a country or region with the widespread transmission have been advised to self-quarantine for 14 days from the time of last possible exposure.
Face masks and respiratory hygiene
The WHO and the US CDC recommend individuals wear non-medical face coverings in public settings where there is an increased risk of transmission and where social distancing measures are difficult to maintain. This recommendation is meant to reduce the spread of the disease by asymptomatic and pre-symptomatic individuals and is complementary to established preventive measures such as social distancing. Face coverings limit the volume and travel distance of expiratory droplets dispersed when talking, breathing, and coughing. Face coverings also filter out particles containing the virus from inhaled air, reducing the chance that the wearer will become infected. Many countries and local jurisdictions encourage or mandate the use of face masks or cloth face coverings by members of the public to limit the spread of the virus.
Masks are also strongly recommended for those who may have been infected and those taking care of someone who may have the disease. When not wearing a mask, the CDC recommends covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available. Proper hand hygiene after any cough or sneeze is encouraged. Healthcare professionals interacting directly with COVID-19 patients are advised to use respirators at least as protective as NIOSH-certified N95 or equivalent, in addition to other personal protective equipment.
Hand-washing and hygiene
When not wearing a mask, the CDC, the WHO, and the NHS recommend covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available. Proper hand hygiene after any cough or sneeze is encouraged. The WHO also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose. The CDC recommends using an alcohol-based hand sanitiser with at least 60% alcohol, but only when soap and water are not readily available. For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ethanol or isopropanol. Hydrogen peroxide is used to help eliminate bacterial spores in the alcohol; it is "not an active substance for hand antisepsis". Glycerol is added as a humectant.
Surface cleaning
Coronaviruses on surfaces die "within hours to days". Coronaviruses die faster when exposed to sunlight and warm temperatures.
Surfaces may be decontaminated with a number of solutions (within one minute of exposure to the disinfectant for a stainless steel surface), including 62–71 percent ethanol, 50–100 percent isopropanol, 0.1 percent sodium hypochlorite, 0.5 percent hydrogen peroxide, and 0.2–7.5 percent povidone-iodine. Other solutions, such as benzalkonium chloride and chlorhexidine gluconate, are less effective. Ultraviolet germicidal irradiation may also be used. The CDC recommends that if a COVID-19 case is suspected or confirmed at a facility such as an office or day care, all areas such as offices, bathrooms, common areas, shared electronic equipment like tablets, touch screens, keyboards, remote controls, and ATM machines used by the ill persons should be disinfected.
Ventilation and air filtration
The WHO recommends ventilation and air filtration in public spaces to help clear out infectious aerosols.
Healthy diet and lifestyle
The Harvard T.H. Chan School of Public Health recommends a healthy diet, being physically active, managing psychological stress, and getting enough sleep. Dark-skinned people are at particular risk of a vitamin D deficiency which can impair the immune system.
Treatment
There is no specific, effective treatment or cure for coronavirus disease 2019 (COVID-19), the disease caused by the SARS-CoV-2 virus. Thus, the cornerstone of management of COVID-19 is supportive care, which includes treatment to relieve symptoms, fluid therapy, oxygen support and prone positioning as needed, and medications or devices to support other affected vital organs.
Most cases of COVID-19 are mild. In these, supportive care includes medication such as paracetamol or NSAIDs to relieve symptoms (fever, body aches, cough), proper intake of fluids, rest, and nasal breathing. Good personal hygiene and a healthy diet are also recommended. The U.S. Centers for Disease Control and Prevention (CDC) recommend that those who suspect they are carrying the virus isolate themselves at home and wear a face mask.
People with more severe cases may need treatment in hospital. In those with low oxygen levels, use of the glucocorticoid dexamethasone is strongly recommended, as it can reduce the risk of death. Noninvasive ventilation and, ultimately, admission to an intensive care unit for mechanical ventilation may be required to support breathing. Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.
Several experimental treatments are being actively studied in clinical trials. Others were thought to be promising early in the pandemic, such as hydroxychloroquine and lopinavir/ritonavir, but later research found them to be ineffective or even harmful. Despite ongoing research, there is still not enough high-quality evidence to recommend so-called early treatment. Nevertheless, in the United States, two monoclonal antibody-based therapies are available for early use in cases thought to be at high risk of progression to severe disease. The antiviral remdesivir is available in the U.S., Canada, Australia, and several other countries, with varying restrictions; however, it is not recommended for people needing mechanical ventilation, and is discouraged altogether by the World Health Organization (WHO), due to limited evidence of its efficacy.Prognosis
The severity of COVID-19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold. In 3-4% of cases (7.4% for those over age 65) symptoms are severe enough to cause hospitalization. Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks. The Italian Istituto Superiore di Sanità reported that the median time between the onset of symptoms and death was twelve days, with seven being hospitalised. However, people transferred to an ICU had a median time of ten days between hospitalisation and death. Prolonged prothrombin time and elevated C-reactive protein levels on admission to the hospital are associated with severe course of COVID-19 and with a transfer to ICU.
Some early studies suggest 10% to 20% of people with COVID-19 will experience symptoms lasting longer than a month. A majority of those who were admitted to hospital with severe disease report long-term problems including fatigue and shortness of breath. On 30 October 2020 WHO chief Tedros Adhanom has warned that "to a significant number of people, the COVID virus poses a range of serious long-term effects". He has described the vast spectrum of COVID-19 symptoms that fluctuate over time as "really concerning." They range from fatigue, a cough and shortness of breath, to inflammation and injury of major organs – including the lungs and heart, and also neurological and psychologic effects. Symptoms often overlap and can affect any system in the body. Infected people have reported cyclical bouts of fatigue, headaches, months of complete exhaustion, mood swings, and other symptoms. Tedros has concluded that therefore herd immunity is "morally unconscionable and unfeasible".
In terms of hospital readmissions about 9% of 106,000 individuals had to return for hospital treatment within 2 months of discharge. The average to readmit was 8 days since first hospital visit. There are several risk factors that have been identified as being a cause of multiple admissions to a hospital facility. Among these are advanced age (above 65 years of age) and presence of a chronic condition such as diabetes, COPD, heart failure or chronic kidney disease.
According to scientific reviews smokers are more likely to require intensive care or die compared to non-smokers, air pollution is similarly associated with risk factors, and pre-existing heart and lung diseases and also obesity contributes to an increased health risk of COVID-19.
It is also assumed that those that are immunocompromised are at higher risk of getting severely sick from SARS-CoV-2. One research that looked into the COVID-19 infections in hospitalized kidney transplant recipients found a mortality rate of 11%.
Children make up a small proportion of reported cases, with about 1% of cases being under 10 years and 4% aged 10–19 years. They are likely to have milder symptoms and a lower chance of severe disease than adults. A European multinational study of hospitalized children published in The Lancet on 25 June 2020 found that about 8% of children admitted to a hospital needed intensive care. Four of those 582 children (0.7%) died, but the actual mortality rate could be "substantially lower" since milder cases that did not seek medical help were not included in the study.
Genetics also plays an important role in the ability to fight off the disease. For instance, those that do not produce detectable type I interferons or produce auto-antibodies against these may get much sicker from COVID-19. Genetic screening is able to detect interferon effector genes.
Pregnant women may be at higher risk of severe COVID-19 infection based on data from other similar viruses, like SARS and MERS, but data for COVID-19 is lacking.
| Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–89 | 90+ |
| Argentina as of 7 May | 0.0 | 0.0 | 0.1 | 0.4 | 1.3 | 3.6 | 12.9 | 18.8 | 28.4 | |
| Australia as of 4 June | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 1.1 | 4.1 | 18.1 | 40.8 |
| Canada as of 3 December | 0.0 | 0.0 | 0.0 | 0.1 | 0.6 | 2.9 | 11.6 | 26.5 | ||
| Alberta as of 3 June | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.2 | 1.9 | 11.9 | 30.8 | |
| Br. Columbia as of 2 June | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.8 | 4.6 | 12.3 | 33.8 | 33.6 |
| Ontario as of 3 June | 0.0 | 0.0 | 0.1 | 0.2 | 0.5 | 1.5 | 5.6 | 17.7 | 26.0 | 33.3 |
| Quebec as of 2 June | 0.0 | 0.1 | 0.1 | 0.2 | 1.1 | 6.1 | 21.4 | 30.4 | 36.1 | |
| Chile as of 31 May | 0.1 | 0.3 | 0.7 | 2.3 | 7.7 | 15.6 | ||||
| China as of 11 February | 0.0 | 0.2 | 0.2 | 0.2 | 0.4 | 1.3 | 3.6 | 8.0 | 14.8 | |
| Colombia as of 3 June | 0.3 | 0.0 | 0.2 | 0.5 | 1.6 | 3.4 | 9.4 | 18.1 | 25.6 | 35.1 |
| Denmark as of 4 June | 0.2 | 4.1 | 16.5 | 28.1 | 48.2 | |||||
| Finland as of 1 December | 0.0 | 0.4 | 1.6 | 9.6 | 32.7 | |||||
| Germany as of 5 June | 0.0 | 0.0 | 0.1 | 1.9 | 19.7 | 31.0 | ||||
| Bavaria as of 5 June | 0.0 | 0.0 | 0.1 | 0.1 | 0.2 | 0.9 | 5.4 | 15.8 | 28.0 | 35.8 |
| Israel as of 3 May | 0.0 | 0.0 | 0.0 | 0.9 | 0.9 | 3.1 | 9.7 | 22.9 | 30.8 | 31.3 |
| Italy as of 3 June | 0.3 | 0.0 | 0.1 | 0.3 | 0.9 | 2.7 | 10.6 | 25.9 | 32.4 | 29.9 |
| Japan as of 7 May | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.6 | 2.5 | 6.8 | 14.8 | |
| Mexico as of 3 June | 3.3 | 0.6 | 1.2 | 2.9 | 7.5 | 15.0 | 25.3 | 33.7 | 40.3 | 40.6 |
| Netherlands as of 3 June | 0.0 | 0.2 | 0.1 | 0.3 | 0.5 | 1.7 | 8.1 | 25.6 | 33.3 | 34.5 |
| Norway as of 1 December | 0.0 | 0.1 | 0.2 | 1.1 | 5.3 | 16.5 | 36.9 | |||
| Philippines as of 4 June | 1.6 | 0.9 | 0.5 | 0.8 | 2.4 | 5.5 | 13.2 | 20.9 | 31.5 | |
| Portugal as of 3 June | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 1.3 | 3.6 | 10.5 | 21.2 | |
| South Africa as of 28 May | 0.3 | 0.1 | 0.1 | 0.4 | 1.1 | 3.8 | 9.2 | 15.0 | 12.3 | |
| South Korea as of 1 December | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 1.2 | 6.4 | 18.2 | |
| Spain as of 29 May | 0.3 | 0.2 | 0.2 | 0.3 | 0.6 | 1.4 | 5.0 | 14.3 | 20.8 | 21.7 |
| Sweden as of 30 November | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 1.9 | 11.6 | 26.2 | 32.9 |
| Switzerland as of 4 June | 0.6 | 0.0 | 0.0 | 0.1 | 0.1 | 0.6 | 3.4 | 11.6 | 28.2 | |
| United States | ||||||||||
| Colorado as of 3 June | 0.2 | 0.2 | 0.2 | 0.2 | 0.8 | 1.9 | 6.2 | 18.5 | 39.0 | |
| Connecticut as of 3 June | 0.2 | 0.1 | 0.1 | 0.3 | 0.7 | 1.8 | 7.0 | 18.0 | 31.2 | |
| Georgia as of 3 June | 0.0 | 0.1 | 0.5 | 0.9 | 2.0 | 6.1 | 13.2 | 22.0 | ||
| Idaho as of 3 June | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 3.1 | 8.9 | 31.4 | |
| Indiana as of 3 June | 0.1 | 0.1 | 0.2 | 0.6 | 1.8 | 7.3 | 17.1 | 30.2 | ||
| Kentucky as of 20 May | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 1.9 | 5.9 | 14.2 | 29.1 | |
| Maryland as of 20 May | 0.0 | 0.1 | 0.2 | 0.3 | 0.7 | 1.9 | 6.1 | 14.6 | 28.8 | |
| Massachusetts as of 20 May | 0.0 | 0.0 | 0.1 | 0.1 | 0.4 | 1.5 | 5.2 | 16.8 | 28.9 | |
| Minnesota as of 13 May | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 1.6 | 5.4 | 26.9 | ||
| Mississippi as of 19 May | 0.0 | 0.1 | 0.5 | 0.9 | 2.1 | 8.1 | 16.1 | 19.4 | 27.2 | |
| Missouri as of 19 May | 0.0 | 0.0 | 0.1 | 0.2 | 0.8 | 2.2 | 6.3 | 14.3 | 22.5 | |
| Nevada as of 20 May | 0.0 | 0.3 | 0.3 | 0.4 | 1.7 | 2.6 | 7.7 | 22.3 | ||
| N. Hampshire as of 12 May | 0.0 | 0.0 | 0.4 | 0.0 | 1.2 | 0.0 | 2.2 | 12.0 | 21.2 | |
| Oregon as of 12 May | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.8 | 5.6 | 12.1 | 28.9 | |
| Texas as of 20 May | 0.0 | 0.5 | 0.4 | 0.3 | 0.8 | 2.1 | 5.5 | 10.1 | 30.6 | |
| Virginia as of 19 May | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 1.0 | 4.4 | 12.9 | 24.9 | |
| Washington as of 10 May | 0.0 | 0.2 | 1.3 | 9.8 | 31.2 | |||||
| Wisconsin as of 20 May | 0.0 | 0.0 | 0.2 | 0.2 | 0.6 | 2.0 | 5.0 | 14.7 | 19.9 | 30.4 |
Complications
Complications may include pneumonia, ARDS, multi-organ failure, septic shock, and death.
Cardiovascular complications may include heart failure, arrhythmias, heart inflammation, and blood clots.
Approximately 20–30% of people who present with COVID-19 have elevated liver enzymes reflecting liver injury.
Neurologic manifestations include seizure, stroke, encephalitis, and Guillain–Barré syndrome (which includes loss of motor functions). Following the infection, children may develop paediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, which can be fatal. In very rare cases, acute encephalopathy can occur, and it can be considered in those who have been diagnosed with COVID-19 and have an altered mental status.
Longer-term effects
Some early studies suggest between 1 in 5 and 1 in 10 people with COVID-19 will experience symptoms lasting longer than a month. A majority of those who were admitted to hospital with severe disease report long-term problems including fatigue and shortness of breath.
On 30 October 2020 WHO chief Tedros has warned that "to a significant number of people, the COVID virus poses a range of serious long-term effects". He has described the vast spectrum of COVID-19 symptoms that fluctuate over time as "really concerning." They range from fatigue, a cough and shortness of breath, to inflammation and injury of major organs – including the lungs and heart, and also neurological and psychologic effects. Symptoms often overlap and can affect any system in the body. Infected people have reported cyclical bouts of fatigue, headaches, months of complete exhaustion, mood swings and other symptoms. Tedros has underlined that therefore the "natural herd immunity" strategy is “morally unconscionable and unfeasible”.
Immunity
The immune response by humans to CoV-2 virus occurs as a combination of the cell-mediated immunity and antibody production, just as with most other infections. Since SARS-CoV-2 has been in the human population only since December 2019, it remains unknown if the immunity is long-lasting in people who recover from the disease. The presence of neutralizing antibodies in blood strongly correlates with protection from infection, but the level of neutralizing antibody declines with time. Those with asymptomatic or mild disease had undetectable levels of neutralizing antibody two months after infection. In another study, the level of neutralizing antibody fell 4 fold 1 to 4 months after the onset of symptoms. However, the lack of antibody in the blood does not mean antibody will not be rapidly produced upon reexposure to SARS-CoV-2. Memory B cells specific for the spike and nucleocapsid proteins of SARS-CoV-2 last for at least 6 months after appearance of symptoms. Nevertheless, 15 cases of reinfection with SARS-CoV-2 have been reported using stringent CDC criteria requiring identification of a different variant from the second infection. There are likely to be many more people who have been reinfected with the virus. Herd immunity will not eliminate the virus if reinfection is common. Some other coronaviruses circulating in people are capable of reinfection after roughly a year.
Mortality
Several measures are commonly used to quantify mortality. These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since the initial outbreak, and population characteristics such as age, sex, and overall health. The mortality rate reflects the number of deaths within a specific demographic group divided by the population of that demographic group. Consequently, the mortality rate reflects the prevalence as well as the severity of the disease within a given population. Mortality rates are highly correlated to age, with relatively low rates for young people and relatively high rates among the elderly.
The case fatality rate (CFR) reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is 2.1% (2,033,641/95,179,173) as of 18 January 2021. The number varies by region. The CFR may not reflect the true severity of the disease, because some infected individuals remain asymptomatic or experience only mild symptoms, and hence such infections may not be included in official case reports. Moreover, the CFR may vary markedly over time and across locations due to the availability of live virus tests.
Infection fatality rate
A key metric in gauging the severity of COVID-19 is the infection fatality rate (IFR), also referred to as the infection fatality ratio or infection fatality risk. This metric is calculated by dividing the total number of deaths from the disease by the total number of infected individuals; hence, in contrast to the CFR, the IFR incorporates asymptomatic and undiagnosed infections as well as reported cases.
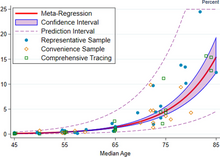

A recent (Dec 2020) systematic review and meta-analysis estimated that population IFR during the first wave of the pandemic was about 0.5% to 1% in many locations (including France, Netherlands, New Zealand, and Portugal), 1% to 2% in other locations (Australia, England, Lithuania, and Spain), and exceeded 2% in Italy. That study also found that most of these differences in IFR reflected corresponding differences in the age composition of the population and age-specific infection rates; in particular, the metaregression estimate of IFR is very low for children and younger adults (e.g., 0.002% at age 10 and 0.01% at age 25) but increases progressively to 0.4% at age 55, 1.4% at age 65, 4.6% at age 75, and 15% at age 85. These results were also highlighted in a December 2020 report issued by the WHO.
Earlier estimates of IFR
At an early stage of the pandemic, the World Health Organization reported estimates of IFR between 0.3% and 1%. On 2 July, The WHO's chief scientist reported that the average IFR estimate presented at a two-day WHO expert forum was about 0.6%. In August, the WHO found that studies incorporating data from broad serology testing in Europe showed IFR estimates converging at approximately 0.5–1%. Firm lower limits of IFRs have been established in a number of locations such as New York City and Bergamo in Italy since the IFR cannot be less than the population fatality rate. As of 10 July, in New York City, with a population of 8.4 million, 23,377 individuals (18,758 confirmed and 4,619 probable) have died with COVID-19 (0.3% of the population). Antibody testing in New York City suggested an IFR of ~0.9%, and ~1.4%. In Bergamo province, 0.6% of the population has died. In September 2020 the U.S. Center for Disease Control & Prevention reported preliminary estimates of age-specific IFRs for public health planning purposes.
Sex differences
Early reviews of epidemiologic data showed greater impact of the pandemic and a higher mortality rate in men in China and Italy. The Chinese Center for Disease Control and Prevention reported the death rate was 2.8% for men and 1.7% for women. Later reviews in June 2020 indicated that there is no significant difference in susceptibility or in CFR between genders. One review acknowledges the different mortality rates in Chinese men, suggesting that it may be attributable to lifestyle choices such as smoking and drinking alcohol rather than genetic factors. Sex-based immunological differences, lesser prevalence of smoking in women and men developing co-morbid conditions such as hypertension at a younger age than women could have contributed to the higher mortality in men. In Europe, 57% of the infected people were men and 72% of those died with COVID-19 were men. As of April 2020, the US government is not tracking sex-related data of COVID-19 infections. Research has shown that viral illnesses like Ebola, HIV, influenza and SARS affect men and women differently.
| Percentage of infected people who are hospitalized | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.1 (0.07–0.2) |
0.5 (0.3–0.8) |
0.9 (0.5–1.5) |
1.3 (0.7–2.1) |
2.6 (1.5–4.2) |
5.1 (2.9–8.3) |
7.8 (4.4–12.8) |
19.3 (10.9–31.6) |
2.6 (1.5–4.3) |
| Male | 0.2 (0.08–0.2) |
0.6 (0.3–0.9) |
1.2 (0.7–1.9) |
1.6 (0.9–2.6) |
3.2 (1.8–5.2) |
6.7 (3.7–10.9) |
11.0 (6.2–17.9) |
37.6 (21.1–61.3) |
3.3 (1.8–5.3) |
| Total | 0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.1 (0.6–1.7) |
1.4 (0.8–2.3) |
2.9 (1.6–4.7) |
5.8 (3.3–9.5) |
9.3 (5.2–15.1) |
26.2 (14.8–42.7) |
2.9 (1.7–4.8) |
| Percentage of hospitalized people who go to Intensive Care Unit | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 16.7 (14.3–19.3) |
8.7 (7.5–9.9) |
11.9 (10.9–13.0) |
16.6 (15.6–17.7) |
20.7 (19.8–21.6) |
23.1 (22.2–24.0) |
18.7 (18.0–19.5) |
4.2 (4.0–4.5) |
14.3 (13.9–14.7) |
| Male | 26.9 (23.1–31.1) |
14.0 (12.2–16.0) |
19.2 (17.6–20.9) |
26.9 (25.4–28.4) |
33.4 (32.0–34.8) |
37.3 (36.0–38.6) |
30.2 (29.1–31.3) |
6.8 (6.5–7.2) |
23.1 (22.6–23.6) |
| Total | 22.2 (19.1–25.7) |
11.6 (10.1–13.2) |
15.9 (14.5–17.3) |
22.2 (21.0–23.5) |
27.6 (26.5–28.7) |
30.8 (29.8–31.8) |
24.9 (24.1–25.8) |
5.6 (5.3–5.9) |
19.0 (18.7–19.44) |
| Percent of hospitalized people who die | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.5 (0.2–1.0) |
0.9 (0.5–1.3) |
1.5 (1.2–1.9) |
2.6 (2.3–3.0) |
5.2 (4.8–5.6) |
10.1 (9.5–10.6) |
16.7 (16.0–17.4) |
25.2 (24.4–26.0) |
14.4 (14.0–14.8) |
| Male | 0.7 (0.3–1.5) |
1.3 (0.8–1.9) |
2.2 (1.7–2.7) |
3.8 (3.3–4.4) |
7.6 (7.0–8.2) |
14.8 (14.1–15.6) |
24.6 (23.7–25.6) |
37.1 (36.1–38.2) |
21.2 (20.8–21.7) |
| Total | 0.6 (0.2–1.3) |
1.1 (0.7–1.6) |
1.9 (1.5–2.3) |
3.3 (2.9–3.8) |
6.5 (6.0–7.0) |
12.6 (12.0–13.2) |
21.0 (20.3–21.7) |
31.6 (30.9–32.4) |
18.1 (17.8–18.4) |
| Percent of infected people who die – infection fatality rate (IFR) | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.001 (<0.001–0.002) |
0.004 (0.002–0.007) |
0.01 (0.007–0.02) |
0.03 (0.02–0.06) |
0.1 (0.08–0.2) |
0.5 (0.3–0.8) |
1.3 (0.7–2.1) |
4.9 (2.7–8.0) |
0.4 (0.2–0.6) |
| Male | 0.001 (<0.001–0.003) |
0.007 (0.003–0.01) |
0.03 (0.02–0.05) |
0.06 (0.03–0.1) |
0.2 (0.1–0.4) |
1.0 (0.6–1.6) |
2.7 (1.5–1.4) |
14.0 (7.9–22.7) |
0.7 (0.4–1.1) |
| Total | 0.001 (<0.001–0.002) |
0.005 (0.003–0.01) |
0.02 (0.01–0.03) |
0.05 (0.03–0.08) |
0.2 (0.1–0.3) |
0.7 (0.4–1.2) |
1.9 (1.1–3.2) |
8.3 (4.7–13.5) |
0.5 (0.3–0.9) |
| Numbers in parentheses are 95% credible intervals for the estimates. | |||||||||
Ethnic differences
In the US, a greater proportion of deaths due to COVID-19 have occurred among African Americans and other minority groups. Structural factors that prevent them from practicing social distancing include their concentration in crowded substandard housing and in "essential" occupations such as retail grocery workers, public transit employees, health-care workers and custodial staff. Greater prevalence of lacking health insurance and care and of underlying conditions such as diabetes, hypertension and heart disease also increase their risk of death. Similar issues affect Native American and Latino communities. According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults. The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water. Leaders have called for efforts to research and address the disparities. In the U.K., a greater proportion of deaths due to COVID-19 have occurred in those of a Black, Asian, and other ethnic minority background. More severe impacts upon victims including the relative incidence of the necessity of hospitalization requirements, and vulnerability to the disease has been associated via DNA analysis to be expressed in genetic variants at chromosomal region 3, features that are associated with European Neanderthal heritage. That structure imposes greater risks that those affected will develop a more severe form of the disease. The findings are from Professor Svante Pääbo and researchers he leads at the Max Planck Institute for Evolutionary Anthropology and the Karolinska Institutet. This admixture of modern human and Neanderthal genes is estimated to have occurred roughly between 50,000 and 60,000 years ago in Southern Europe.
Comorbidities
Most of those who die of COVID-19 have pre-existing (underlying) conditions, including hypertension, diabetes mellitus, and cardiovascular disease. According to March data from the United States, 89% of those hospitalised had preexisting conditions. The Italian Istituto Superiore di Sanità reported that out of 8.8% of deaths where medical charts were available, 96.1% of people had at least one comorbidity with the average person having 3.4 diseases. According to this report the most common comorbidities are hypertension (66% of deaths), type 2 diabetes (29.8% of deaths), ischemic heart disease (27.6% of deaths), atrial fibrillation (23.1% of deaths) and chronic renal failure (20.2% of deaths).
Most critical respiratory comorbidities according to the CDC, are: moderate or severe asthma, pre-existing COPD, pulmonary fibrosis, cystic fibrosis. Evidence stemming from meta-analysis of several smaller research papers also suggests that smoking can be associated with worse patient outcomes. When someone with existing respiratory problems is infected with COVID-19, they might be at greater risk for severe symptoms. COVID-19 also poses a greater risk to people who misuse opioids and methamphetamines, insofar as their drug use may have caused lung damage.
In August 2020 the CDC issued a caution that tuberculosis infections could increase the risk of severe illness or death. The WHO recommended that patients with respiratory symptoms be screened for both diseases, as testing positive for COVID-19 couldn't rule out co-infections. Some projections have estimated that reduced TB detection due to the pandemic could result in 6.3 million additional TB cases and 1.4 million TB related deaths by 2025.
Name
During the initial outbreak in Wuhan, China, the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus", with the disease sometimes called "Wuhan pneumonia". In the past, many diseases have been named after geographical locations, such as the Spanish flu, Middle East Respiratory Syndrome, and Zika virus.
In January 2020, the WHO recommended 2019-nCov and 2019-nCoV acute respiratory disease as interim names for the virus and disease per 2015 guidance and international guidelines against using geographical locations (e.g. Wuhan, China), animal species, or groups of people in disease and virus names in part to prevent social stigma.
The official names COVID-19 and SARS-CoV-2 were issued by the WHO on 11 February 2020. Tedros Adhanom explained: CO for corona, VI for virus, D for disease and 19 for when the outbreak was first identified (31 December 2019). The WHO additionally uses "the COVID-19 virus" and "the virus responsible for COVID-19" in public communications.
History
The virus is thought to be natural and has an animal origin, through spillover infection. There are several theories about where the first case (the so-called patient zero) originated. Phylogenetics estimates that SARS-CoV-2 arose in October or November 2019. Evidence suggests that it descends from a coronavirus that infects wild bats and spread to humans through an intermediary wildlife host.
The first known human infections were in Wuhan, Hubei, China. A study of the first 41 cases of confirmed COVID-19, published in January 2020 in The Lancet, reported the earliest date of onset of symptoms as 1 December 2019. Official publications from the WHO reported the earliest onset of symptoms as 8 December 2019. Human-to-human transmission was confirmed by the WHO and Chinese authorities by 20 January 2020. According to official Chinese sources, these were mostly linked to the Huanan Seafood Wholesale Market, which also sold live animals. In May 2020, George Gao, the director of the CDC, said animal samples collected from the seafood market had tested negative for the virus, indicating that the market was the site of an early superspreading event, but it was not the site of the initial outbreak. Traces of the virus have been found in wastewater that was collected from Milan and Turin, Italy, on 18 December 2019.
By December 2019, the spread of infection was almost entirely driven by human-to-human transmission. The number of coronavirus cases in Hubei gradually increased, reaching 60 by 20 December and at least 266 by 31 December. On 24 December, Wuhan Central Hospital sent a bronchoalveolar lavage fluid (BAL) sample from an unresolved clinical case to sequencing company Vision Medicals. On 27 and 28 December, Vision Medicals informed the Wuhan Central Hospital and the Chinese CDC of the results of the test, showing a new coronavirus. A pneumonia cluster of unknown cause was observed on 26 December and treated by the doctor Zhang Jixian in Hubei Provincial Hospital, who informed the Wuhan Jianghan CDC on 27 December. On 30 December, a test report addressed to Wuhan Central Hospital, from company CapitalBio Medlab, stated an erroneous positive result for SARS, causing a group of doctors at Wuhan Central Hospital to alert their colleagues and relevant hospital authorities of the result. That evening, the Wuhan Municipal Health Commission issued a notice to various medical institutions on "the treatment of pneumonia of unknown cause". Eight of these doctors, including Li Wenliang (punished on 3 January), were later admonished by the police for spreading false rumours, and another, Ai Fen, was reprimanded by her superiors for raising the alarm.
The Wuhan Municipal Health Commission made the first public announcement of a pneumonia outbreak of unknown cause on 31 December, confirming 27 cases—enough to trigger an investigation.
During the early stages of the outbreak, the number of cases doubled approximately every seven and a half days. In early and mid-January 2020, the virus spread to other Chinese provinces, helped by the Chinese New Year migration and Wuhan being a transport hub and major rail interchange. On 20 January, China reported nearly 140 new cases in one day, including two people in Beijing and one in Shenzhen. Later official data shows 6,174 people had already developed symptoms by then, and more may have been infected. A report in The Lancet on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential". On 30 January, the WHO declared the coronavirus a Public Health Emergency of International Concern. By this time, the outbreak spread by a factor of 100 to 200 times.
On 31 January 2020, Italy had its first confirmed cases, two tourists from China. As of 13 March 2020, the WHO considered Europe the active centre of the pandemic. On 19 March 2020, Italy overtook China as the country with the most deaths. By 26 March, the United States had overtaken China and Italy with the highest number of confirmed cases in the world. Research on coronavirus genomes indicates the majority of COVID-19 cases in New York came from European travellers, rather than directly from China or any other Asian country. Retesting of prior samples found a person in France who had the virus on 27 December 2019 and a person in the United States who died from the disease on 6 February 2020.
On 11 June 2020, after 55 days without a locally transmitted case, Beijing reported the first COVID-19 case, followed by two more cases on 12 June. By 15 June 79 cases were officially confirmed. Most of these patients went to Xinfadi Wholesale Market.
RT-PCR testing of untreated wastewater samples from Brazil and Italy have suggested detection of SARS-CoV-2 as early as November and December 2019, respectively, but the methods of such sewage studies have not been optimised, many have not been peer reviewed, details are often missing, and there is a risk of false positives due to contamination or if only one gene target is detected. A September 2020 review journal article said, "The possibility that the COVID-19 infection had already spread to Europe at the end of last year is now indicated by abundant, even if partially circumstantial, evidence", including pneumonia case numbers and radiology in France and Italy in November and December.
Misinformation
After the initial outbreak of COVID-19, misinformation and disinformation regarding the origin, scale, prevention, treatment, and other aspects of the disease rapidly spread online.
In September 2020, the U.S. CDC published preliminary estimates of the risk of death by age groups in the United States, but those estimates were widely misreported and misunderstood.
Other animals
Humans appear to be capable of spreading the virus to some other animals, a type of disease transmission referred to as zooanthroponosis. A domestic cat in Liège, Belgium, tested positive after it started showing symptoms (diarrhoea, vomiting, shortness of breath) a week later than its owner, who was also positive. Tigers and lions at the Bronx Zoo in New York, United States, tested positive for the virus and showed symptoms of COVID-19, including a dry cough and loss of appetite. Minks at two farms in the Netherlands also tested positive for COVID-19. In Denmark, as of October 31, 2020, 175 mink farms had seen COVID-19 infection in mink, and also USA; Finland, Sweden and Spain have seen infections in mink.
A study on domesticated animals inoculated with the virus found that cats and ferrets appear to be "highly susceptible" to the disease, while dogs appear to be less susceptible, with lower levels of viral replication. The study failed to find evidence of viral replication in pigs, ducks, and chickens. It is unknown if other great ape species can be infected with COVID-19, though many primate sanctuaries presume transmission from humans to other apes is possible, as it is for other respiratory viruses.
As of August 2020, dozens of domestic cats and dogs had tested positive, though according to the U.S. CDC, there was no evidence they transmitted the virus to humans. CDC guidance recommends potentially infected people avoid close contact with pets.
On 4 November 2020, Prime Minister of Denmark Mette Frederiksen stated that a mutated coronavirus was being transmitted to humans via minks, tied primarily to mink farms in Northern Jutland.
Research
International research on vaccines and medicines in COVID-19 is underway by government organisations, academic groups, and industry researchers. There has been a great deal of COVID-19 research, involving accelerated research processes and publishing shortcuts to meet the global demand.
As of December 2020[update], hundreds of clinical trials have been undertaken, with research happening on every continent except Antarctica. As of November 2020[update], more than 200 possible treatments had been studied in humans so far.
Transmission and prevention research
Modelling research has been conducted with several objectives, including predictions of the dynamics of transmission, diagnosis and prognosis of infection, estimation of the impact of interventions, or allocation of resources. Modelling studies are mostly based on epidemiological models, estimating the number of infected people over time under given conditions. Several other types of models have been developed and used during the COVID-19 including computational fluid dynamics models to study the flow physics of COVID-19, retrofits of crowd movement models to study occupant exposure, mobility-data based models to investigate transmission, or the use of macroeconomic models to assess the economic impact of the pandemic. Further, conceptual frameworks from crisis management research have been applied to better understand the effects of COVID-19 on organizations worldwide.
Repurposed antiviral drugs make up most of the research into COVID-19 treatments. Other candidates in trials include vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinant angiotensin-converting enzyme 2.
In March 2020, the World Health Organization initiated the Solidarity Trial to assess the treatment effects of some promising drugs: an experimental drug called remdesivir; anti-malarial drugs chloroquine and hydroxychloroquine; two anti-HIV drugs, lopinavir/ritonavir; and interferon-beta. More than 300 active clinical trials were underway as of April 2020.
Research on the antimalarial drugs hydroxychloroquine and chloroquine showed that it was ineffective at best, and that it may reduce the antiviral activity of remdesivir. By May 2020[update], France, Italy and Belgium had banned the use of hydroxychloroquine as a COVID-19 treatment.
In June, initial results from a randomised trial in the United Kingdom showed that dexamethasone reduced mortality by one third for patients who are critically ill on ventilators and one fifth for those receiving supplemental oxygen. Because this is a well tested and widely available treatment, it was welcomed by the WHO, which is in the process of updating treatment guidelines to include dexamethasone and other steroids. Based on those preliminary results, dexamethasone treatment has been recommended by the NIH for patients with COVID-19 who are mechanically ventilated or who require supplemental oxygen but not in patients with COVID-19 who do not require supplemental oxygen.
In September 2020, the WHO released updated guidance on using corticosteroids for COVID-19. The WHO recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of people with severe and critical COVID-19 (strong recommendation, based on moderate certainty evidence). The WHO suggests not to use corticosteroids in the treatment of people with non-severe COVID-19 (conditional recommendation, based on low certainty evidence). The updated guidance was based on a meta-analysis of clinical trials of critically ill COVID-19 patients.
In September 2020, the EMA endorsed the use of dexamethasone in adults and adolescents from twelve years of age and weighing at least 40 kilograms (88 lb) who require supplemental oxygen therapy. Dexamethasone can be taken by mouth or given as an injection or infusion (drip) into a vein.
In November 2020, the FDA issued an emergency use authorization for the investigational monoclonal antibody therapy bamlanivimab for the treatment of mild-to-moderate COVID-19. Bamlanivimab is authorized for people with positive results of direct SARS-CoV-2 viral testing who are twelve years of age and older weighing at least 40 kilograms (88 lb), and who are at high risk for progressing to severe COVID-19 or hospitalization. This includes those who are 65 years of age or older, or who have certain chronic medical conditions.
Cytokine storm
A cytokine storm can be a complication in the later stages of severe COVID-19. A cytokine storm is a potentially deadly immune reaction where a large amount of pro-inflammatory cytokines and chemokines are released too quickly; A cytokine storm can lead to ARDS and multiple organ failure. Data collected from Jin Yin-tan Hospital in Wuhan, China indicates that patients who had more severe responses to COVID-19 had greater amounts of pro-inflammatory cytokines and chemokines in their system than patients who had milder responses; These high levels of pro-inflammatory cytokines and chemokines indicate presence of a cytokine storm.
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed. It is undergoing a Phase II non-randomised trial at the national level in Italy after showing positive results in people with severe disease. Combined with a serum ferritin blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with cytokine release syndrome), it is meant to counter such developments, which are thought to be the cause of death in some affected people. The interleukin-6 receptor antagonist was approved by the FDA to undergo a Phase III clinical trial assessing its effectiveness on COVID-19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017. To date, there is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the blood-brain barrier, and exacerbating neurotoxicity while having no effect on the incidence of CRS.
Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T-cells in hospitalised patients with COVID-19.
The Feinstein Institute of Northwell Health announced in March a study on "a human antibody that may prevent the activity" of IL-6.
Passive antibodies
Transferring purified and concentrated antibodies produced by the immune systems of those who have recovered from COVID-19 to people who need them is being investigated as a non-vaccine method of passive immunisation. Viral neutralization is the anticipated mechanism of action by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralizing antibodies. As of 8 August 2020, eight neutralizing antibodies targeting the spike protein of SARS-CoV-2 have entered clinical studies. It has been proposed that selection of broad-neutralizing antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID-19 but also future SARS-related CoV infections. Other mechanisms, however, such as antibody-dependent cellular cytotoxicity and/or phagocytosis, may be possible. Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.
The use of passive antibodies to treat people with active COVID-19 is also being studied. This involves the production of convalescent serum, which consists of the liquid portion of the blood from recovered patients and contains antibodies specific to this virus, which is then administered to current patients. This strategy was tried for SARS with inconclusive results. A Cochrane review in October 2020 found insufficient evidence to recommend for or against this treatment in COVID-19, due in large part to the methodology of the clinical trials conducted so far. Specifically, there are no trials yet conducted for which the safety of convalescent serum administration to people with COVID-19 can be determined, and the differing outcomes measured in different studies limits their use in determining efficacy.
Laminoid antibodies
Research using alpacas and llamas in Peru may produce a treatment for COVID-19. Alpacas and other laminoid animals (South American camel-like animals) naturally produce a very small type of antibody known as nanobodies.
See also
![]() Medicine portal
Medicine portal
- Coronavirus diseases, a group of closely related syndromes
- Disease X, a WHO term
Notes
- ^ Known as "close contact" which is variously defined, including within ~1.8 metres (six feet) by the US Centers for Disease Control and Prevention (CDC), and being face to face for a cumulative total of 15 minutes, or either 15 minutes of face to face proximity or sharing an enclosed space for a prolonged period such as two hours by the Australian Health Department.








