Prader-Willi Syndrome
Summary
Clinical characteristics.
Prader-Willi syndrome (PWS) is characterized by severe hypotonia and feeding difficulties in early infancy, followed in later infancy or early childhood by excessive eating and gradual development of morbid obesity (unless eating is externally controlled). Motor milestones and language development are delayed. All individuals have some degree of cognitive impairment. A distinctive behavioral phenotype (with temper tantrums, stubbornness, manipulative behavior, and obsessive-compulsive characteristics) is common. Hypogonadism is present in both males and females and manifests as genital hypoplasia, incomplete pubertal development, and, in most, infertility. Short stature is common (if not treated with growth hormone); characteristic facial features, strabismus, and scoliosis are often present.
Diagnosis/testing.
Consensus clinical diagnostic criteria are accurate, but the mainstay of diagnosis is DNA methylation testing to detect abnormal parent-specific imprinting within the Prader-Willi critical region (PWCR) on chromosome 15; this testing determines whether the region is maternally inherited only (i.e., the paternally contributed region is absent) and detects more than 99% of affected individuals. DNA methylation-specific testing is important to confirm the diagnosis of PWS in all individuals, but especially in those who have atypical findings or are too young to manifest sufficient features to make the diagnosis on clinical grounds.
Management.
Treatment of manifestations: In infancy, special nipples or enteral tube feeding to assure adequate nutrition; physical therapy may improve muscle strength; hormonal and surgical treatments can be considered for cryptorchidism. In childhood, strict supervision of daily food intake based on height, weight, and body mass index (BMI) to provide energy requirements while limiting excessive weight gain (keeping BMI Z score <2 or better) and encouraging physical activity. Growth hormone replacement therapy to normalize height, increase lean body mass and mobility, and decrease fat mass. Evaluation and treatment of sleep disturbance per the general population. Educational planning should be instigated and speech therapy provided if needed. Firm limit-setting to manage behavioral problems; serotonin reuptake inhibitors are helpful for most teenagers and adults. Replacement of sex hormones at puberty produces adequate secondary sexual characteristics. N-acetylcysteine or topiramate may help reduce skin picking. Modafinil has been successful in treating daytime sleepiness in many children. In adulthood, a group home for individuals with PWS that regulates behavior and weight management may prevent morbid obesity, and growth hormone may help to maintain muscle bulk.
Prevention of secondary complications: Weight control to avoid development of type 2 diabetes mellitus; calcium and vitamin D supplementation to avoid osteoporosis; if osteoporosis develops, consider treatment with a bisphosphonate.
Surveillance: Infants should be screened for strabismus; routine monitoring of height, weight, and BMI to assure appropriateness of exercise program and diet; annual testing for hypothyroidism.
Other: No medications are currently known to aid in controlling hyperphagia, although several are under investigation.
Genetic counseling.
PWS is caused by an absence of expression of imprinted genes in the paternally derived PWS/Angelman syndrome (AS) region (i.e., 15q11.2-q13) of chromosome 15 by one of several genetic mechanisms (paternal deletion, maternal uniparental disomy 15 and rarely an imprinting defect). The risk to the sibs of an affected child of having PWS depends on the genetic mechanism that resulted in the absence of expression of the paternally contributed 15q11.2-q13 region. The risk to sibs is typically less than 1% if the affected child has a deletion or uniparental disomy, up to 50% if the affected child has an imprinting defect, and up to 25% if a parental chromosome translocation is present. Prenatal testing is possible for pregnancies at increased risk if the underlying genetic mechanism is known.
Diagnosis
Consensus diagnostic criteria for Prader-Willi syndrome (PWS) developed in 1993 [Holm et al 1993] have proven to be accurate [Gunay-Aygun et al 2001] and continue to be useful for the clinician. However, confirmation of the diagnosis requires molecular genetic testing, which was not widely available when the criteria were developed.
Suggestive Findings
Prader-Willi syndrome should be suspected in individuals with the specific clinical findings listed below or findings on cytogenetic/FISH/chromosomal microarray.
Clinical findings that should prompt diagnostic testing have been proposed based on analysis of diagnostic criteria met in individuals in whom the diagnosis of PWS has been molecularly confirmed [Gunay-Aygun et al 2001]. These differ by age group. The presence of all of the following findings at the age indicated is sufficient to justify DNA methylation analysis for PWS (see Establishing the Diagnosis):
Birth to age two years
- Hypotonia with poor suck (neonatal period)
Age two to six years
- Hypotonia with history of poor suck
- Global developmental delay
Age six to 12 years
- History of hypotonia with poor suck (hypotonia often persists)
- Global developmental delay
- Excessive eating with central obesity if uncontrolled
Age 13 years to adulthood
- Cognitive impairment, usually mild intellectual disability
- Excessive eating with central obesity if uncontrolled
- Hypothalamic hypogonadism and/or typical behavior problems
Cytogenetic/FISH/chromosomal microarray findings. Approximately 70% of individuals with PWS have a deletion on one chromosome 15 involving bands 15q11.2-q13, which can be detected using high-resolution chromosome studies and fluorescence in situ hybridization (FISH) testing or chromosomal microarray.
Note: The typical deletion is one of two sizes: extending from the distal breakpoint (BP3) to one of two proximal breakpoints (BP1 or BP2). Clinical FISH testing detects both of these deletions and typically will not distinguish between them. Additional atypical and unique deletions occur in approximately 8% of deletion cases [Kim et al 2012].
Approximately 1% of affected individuals have a detectable chromosome rearrangement (i.e., translocation or inversion) resulting in a deletion of bands 15q11.2-q13.
Fewer than 1% of individuals have a "balanced" chromosome rearrangement breaking within 15q11.2-q13 and detectable by chromosome analysis and FISH.
Establishing the Diagnosis
The diagnosis of PWS is established in a proband with DNA methylation analysis demonstrating abnormal parent-specific imprinting within the Prader-Willi critical region (PWCR) on chromosome 15 in which the region demonstrates maternal-only imprinting (i.e., the absence of paternal-only expressed genes).
Three main molecular mechanisms that result in PWS include paternal deletion, maternal uniparental disomy (UPD) 15 and imprinting defect (ID). DNA methylation analysis is the only technique that will diagnose PWS caused by all three genetic mechanisms as well as differentiate PWS from Angelman syndrome (AS) in deletion cases [Glenn et al 1996, Kubota et al 1996, Glenn et al 1997].
See Table 1 for a summary of molecular genetic testing used to define the underlying genetic mechanism in a proband.
The main molecular mechanisms are further classified by specific genetic etiology into molecular class Ia-IIIb, which is important for genetic counseling (see Table 3).
See Figure 1 for a comprehensive testing strategy to establish the genetic mechanism of an individual with DNA methylation analysis consistent with PWS.

Figure 1.
Comprehensive testing strategy to establish the molecular class of PWS FISH = fluorescence in situ hybridization
Note: A DNA methylation analysis consistent with PWS is sufficient for clinical diagnosis but not for genetic counseling, which requires identification of the underlying genetic mechanism (Table 1). See Genetic Counseling.
Table 1.
Testing Used in Prader-Willi Syndrome
| Method | Genetic Mechanisms Detected 1 | Proportion of PWS Detected by Method |
|---|---|---|
| DNA methylation 2 | Deletions, UPD & ID | >99% |
| MS-MLPA 3 | Deletions, UPD & ID | >99% |
| FISH 4 | Deletions | 65%-75% |
| CMA 5 | Deletions | 65%-75% |
| CMA-SNP array 6 | Deletions & some UPDs | 80%-90% |
| DNA polymorphisms 7 | UPD and ID | 20%-30% |
| DNA sequence 8 | ID with IC deletions | <1% |
IC = imprinting center; ID = imprinting defect; UPD = uniparental disomy
- 1.
See Molecular Genetics for more details.
- 2.
Can establish diagnosis, but will not distinguish genetic mechanism; can be done by Southern blot or methylation-specific PCR.
- 3.
Methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA):
• Will distinguish deletion from disomy (UPD and ID);
• Will not distinguish UPD from ID;
• Can give approximate size of deletion and identify type 1 and 2 deletions (see Figure 2);
• Can also detect most IC and SNORD116 microdeletions (see Figure 2 and Molecular Pathogenesis).- 4.
FISH is typically done in conjunction with a karyotype. Information is limited to AS/PWS region and the specific probes used (e.g., SNRPN). FISH does not query the whole AS/PWS region and will miss small deletions. It does not give information about the rest of the chromosomes, and does not distinguishbetween normal, UPD, and ID.
- 5.
Chromosomal microarray (CMA) has a slightly higher detection frequency than FISH and will provide detailed information regarding size of the deletion. Also, it gives information regarding deletions and duplication in the remainder of the genome. CMA is far more precise than karyotype and FISH; it will detect SNORD116 microdeletions (see Figure 2 and Molecular Pathogenesis).
- 6.
Same as CMA; in addition, use of small nucleotide polymorphisms (SNP) will allow detection of UPD in cases with long stretches of homozygosity.
- 7.
Not a first line test; performed after DNA methylation analysis diagnoses PWS, but FISH or CMA analysis indicates disomy.
- 8.
DNA sequencing has a very specific role in IDs to distinguish IC deletions from epimutations. It is limited to a region of <4.3 kb in the PWS IC smallest region of deletion overlap (SRO) [Ohta et al 1999]. The map location is approximately 25,196,494 to 25,200,794 bp [UCSC Genome Browser, hg19].
Clinical Characteristics
Clinical Description
Perinatal. Fetal size is generally within the normal range. Prenatal hypotonia usually results in decreased fetal movement, abnormal fetal position at delivery, and increased incidence of assisted delivery or cesarean section. Birth weight and body mass index (BMI) are on average 15% less than in normal sibs [Miller et al 2011].
Hypotonia. Infantile hypotonia is a nearly universal finding, causing decreased movement and lethargy with decreased spontaneous arousal, weak cry, and poor reflexes, including poor suck. The hypotonia is central in origin, and neuromuscular studies including muscle biopsy, when done for diagnostic purposes, are generally normal or show nonspecific signs of disuse.
The poor suck, lethargy, and poor appetite result in failure to thrive in early infancy, and enteral tube feeding or the use of special nipples is generally required for a variable period of time, usually weeks to months. By the time that the child is drinking from a cup or eating solids, a period of approximately normal eating behavior occurs.
The hypotonia improves over time. Adults remain mildly hypotonic with decreased muscle bulk and tone.
Developmental delay. Delayed motor development is present in 90%-100% of children with PWS, with average early milestones achieved at about double the normal age (e.g., sitting at 12 months, walking at 24 months). Language milestones are also typically delayed. Intellectual disabilities are generally evident by the time the child reaches preschool age. Testing indicates that most persons with PWS fall in the mildly intellectually disabled range (mean IQ: 60s to 70s), with approximately 40% having borderline disability or low-normal intelligence and approximately 20% having moderate disability. Regardless of measured IQ, most children with PWS have multiple severe learning disabilities and poor academic performance for their intellectual abilities [Whittington et al 2004a]. Although a small proportion of affected individuals have extremely impaired language development, verbal ability is a relative strength for most. Based on the authors' experiences, a small percentage of individuals with PWS are able to attend and graduate from college.
Hypogonadism. In both sexes, hypogonadism is present and manifests as genital hypoplasia, incomplete pubertal development, and infertility in the vast majority. Genital hypoplasia is evident at birth and throughout life.
- Males. The penis may be small, and most characteristic is a hypoplastic scrotum that is small, poorly rugated, and poorly pigmented. Unilateral or bilateral cryptorchidism is present in 80%-90% of males.
- Females. The genital hypoplasia is often overlooked; however, the labia majora and minora and the clitoris are generally small from birth.
The hypogonadism is usually associated with low serum concentration of gonadotropins and causes incomplete, delayed, and sometimes disordered pubertal development. Precocious adrenarche occurs in approximately 15%-20%. Infertility is the rule, although a few instances of reproduction in females have been reported [Akefeldt et al 1999; Schulze et al 2001; Vats & Cassidy, unpublished data]. Although the hypogonadism in PWS has long been believed to be entirely hypothalamic in origin, recent studies have suggested a combination of hypothalamic and primary gonadal deficiencies [Eldar-Geva et al 2009, Hirsch et al 2009, Eldar-Geva et al 2010, Gross-Tsur et al 2012], a conclusion largely based on the absence of hypogonadotropism and abnormally low inhibin B levels in some affected individuals of both sexes.
In one study of 84 individuals with PWS (half males, half females) age two to 35 years [Crinò et al 2003], the following were identified:
- Males. Cryptorchidism 100%, small testes 76%, scrotal hypoplasia 69%
- Females. Labia minora and/or clitoral hypoplasia 76%, primary amenorrhea 56%, spontaneous menarche (mostly spotting) 44% of those older than age 15 years
- Both sexes. Premature pubarche 14%, precocious puberty 3.6% (1 male, 2 females)
Appetite and obesity. In contrast to the long-held view that there are only two distinct nutritional phases in PWS (i.e., failure to thrive followed by hyperphagia leading to obesity) a multicenter study [Miller et al 2011] found that the transition between nutritional phases is much more complex, with seven different nutritional phases through which individuals with PWS typically progress (Table 2).
Table 2.
Nutritional Phases in PWS
| Phase | Median Ages | Clinical Characteristics |
|---|---|---|
| 0 | Prenatal - birth | Decreased fetal movements & lower birth weight than sibs |
| 1a | 0-9 months | Hypotonia with difficulty feeding & decreased appetite |
| 1b | 9-25 months | Improved feeding & appetite; growing appropriately |
| 2a | 2.1-4.5 years | Weight increasing without appetite increase or excess calories |
| 2b | 4.5-8 years | Increased appetite & calories, but can feel full |
| 3 | 8 years - adulthood | Hyperphagic, rarely feels full |
| 4 | Adulthood | Appetite no longer insatiable for some |
Miller et al [2011]
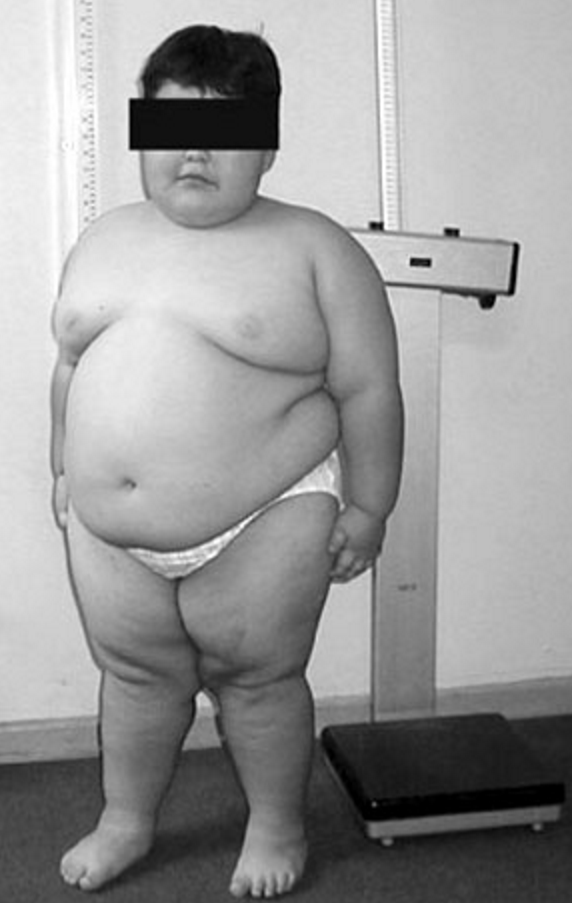
The hyperphagia that occurs in PWS is believed to be caused by a hypothalamic abnormality resulting in lack of satiety. Food-seeking behavior, with hoarding or foraging for food, eating of inedibles, and stealing of food or money to buy food, are common. In most, gastric emptying is delayed, and vomiting is rare. Obesity results from these behaviors and from decreased total caloric requirement. The latter is due to decreased resting energy expenditure resulting from decreased activity and decreased lean body mass (primarily muscle) compared with unaffected individuals. The obesity in PWS is primarily central (abdomen, buttocks, and thighs) in both sexes, and interestingly, there is less visceral fat in obese individuals than would be expected for the degree of obesity. Obesity and its complications are the major causes of morbidity and mortality (see Morbidity and mortality).
Several independent groups have shown that ghrelin levels are significantly elevated in hyperphagic older children and adults with PWS before and after meals [Cummings et al 2002, DelParigi et al 2002, Haqq et al 2003b]. Ghrelin is a potent circulating orexigenic hormone that is produced mainly in the stomach. Circulating ghrelin levels rise after fasting and are suppressed by food intake. The appetite-inducing effect acts through the appetite regulating pathway in the hypothalamus. Ghrelin levels are lower in non-PWS obese individuals than in lean controls, and they decrease with age [Scerif et al 2011].
A small study of nine non-hyperphagic children with PWS (age 17-60 months) found similar levels of circulating ghrelin as in the eight control children matched for BMI, age, and sex [Erdie-Lalena et al 2006]. By contrast, in two much larger and younger study cohorts of children and adolescents with PWS ghrelin levels were significantly elevated in the PWS group at any age compared to controls [Feigerlová et al 2008, Kweh et al 2015]. In fact, the highest ghrelin levels in PWS were found in the youngest children. Thus, in these two large studies the hyperghrelinemia occurred at an age long before the development of obesity and increased appetite in PWS. Furthermore, several groups have now shown that pharmacologic reduction of ghrelin to normal levels in PWS, using either short- or long-acting agents, did not affect the weight, appetite, or eating behavior in hyperphagic individuals [Haqq et al 2003a, Tan et al 2004, De Waele et al 2008]. At this time there are no consistently identified hormonal abnormalities to explain the hyperphagia, and the metabolic correlates of hyperphagia in PWS remain uncertain.
Endocrinologic concerns. Up to 25% of adults with PWS (particularly those with significant obesity) have type 2 diabetes [Butler et al 2002] with a mean age of onset of 20 years. In the last 15 years, earlier diagnosis and education of parents, use of growth hormone therapy, and the frequency of group homes specific for PWS have led to reduction in the development of morbid obesity (and as a result, in type 2 diabetes) among individuals with PWS.
Central hypothyroidism, with a normal thyroid-stimulating hormone value and low free thyroxine level, has been documented in up to 25% of individuals with PWS, with a mean age of diagnosis and treatment of two years [Miller et al 2008, Diene et al 2010].
Central adrenal insufficiency (CAI) following overnight single-dose metyrapone tests was noted in 60% of children with PWS in one study, suggesting that this may be the cause of the high incidence of sudden death in this population [de Lind van Wijngaarden et al 2008]. It is known that introducing GH therapy can precipitate adrenal crisis in individuals with incipient adrenal insufficiency by accelerating the peripheral metabolism of cortisol, which may explain the correlation between the incidence of sudden death at the beginning of GH treatment and CAI in individuals with PWS [Scaroni et al 2008]. However, subsequent studies have found normal cortisol responses to low- and high-dose synacthen testing, as well as to insulin tolerance testing [Nyunt et al 2010, Farholt et al 2011]; thus, whether CAI is a true issue for individuals with PWS remains uncertain at this time and no consensus exists among endocrinologists as to whether evaluation for CAI should be performed on every individual with PWS or only those with symptoms consistent with adrenal insufficiency.
Sleep abnormalities are well documented and include reduced REM (rapid eye movement) latency, altered sleep architecture, oxygen desaturation, and both central and obstructive apnea [Festen et al 2006, Priano et al 2006]. Primary hypothalamic dysfunction is thought to be the cause of the alterations in sleep microstructure and abnormalities in ventilation during sleep, with studies showing low levels of orexin and hypocretin in the cerebrospinal fluid and decreased levels of acetyl-cholinergic neurons in the pedunculo-pontine tegmental nucleus [Dauvilliers et al 2003, Nevsimalova et al 2005, Bruni et al 2010, Hayashi et al 2011]. Some individuals with PWS have excessive daytime sleepiness, which resembles narcolepsy, with rapid onset of REM sleep and decrease in non-REM sleep instability [Bruni et al 2010].
Behavior. A characteristic behavior profile with temper tantrums, stubbornness, controlling and manipulative behavior, compulsivity, and difficulty with change in routine becomes evident in early childhood in 70%-90% of individuals with PWS.
- Many of the behavioral characteristics are suggestive of autism; one study showed that 19% of 59 individuals with PWS versus 15% of age-, sex-, and IQ-matched controls satisfy diagnostic criteria for autism [Descheemaeker et al 2006].
- In another study of 58 children, attention deficit/hyperactivity symptoms and insistence on sameness were common and of early onset [Wigren & Hansen 2005].
- This behavior disorder has been reported to increase with age and body mass index (BMI) [Steinhausen et al 2004], although it diminishes considerably in older adults [Dykens 2004].
- Psychosis is evident by young adulthood in 10%-20% of affected individuals, and is more frequent in those with UPD [Boer et al 2002, Clarke et al 2002, Vogels et al 2004, Yang et al 2013].
Behavioral and psychiatric problems interfere most with the quality of life in adolescence and adulthood.
Growth. Short stature, if not apparent in childhood, is almost always present during the second decade in the absence of growth hormone (GH) replacement, and lack of a pubertal growth spurt results in an average untreated height of 155 cm for males and 148 cm for females. The hands and feet grow slowly and are generally below the fifth centile by age ten years, with an average adult female foot size of 20.3 cm and average adult male foot size of 22.3 cm. Growth charts for affected infants and children not treated with growth hormone have been published [Butler et al 2011, Butler et al 2015] and growth charts for growth hormone-treated children with PWS have been developed [MG Butler & DJ Driscoll, unpublished data].
Data from at least 15 studies involving more than 300 affected children [Burman et al 2001] document reduced GH secretion in PWS. GH deficiency is also seen in adults with PWS [Grugni et al 2006, Höybye 2007].
Dysmorphic features. Characteristic facial features (narrow bifrontal diameter, almond-shaped palpebral fissures, narrow nasal bridge, thin vermilion of the upper lip with down-turned corners of the mouth) may or may not be apparent at birth and slowly evolve over time.
Hypopigmentation of hair, eyes, and skin is frequently found in patients with a deletion due to loss of a single copy of the gene OCA2.
Ophthalmic issues. Strabismus is seen in 60%-70%.
Skeletal findings. Hip dysplasia occurs in approximately 10%-20% [West & Ballock 2004, Shim et al 2010]. Bone fractures are a risk due to the increased frequency of osteopenia and osteoporosis.
Scoliosis, present in 40%-80%, varies in age of onset and severity.
Other. Rates of the following are increased:
- Possibility of recurrent respiratory infections (in ≤50% of individuals)
- Leg edema and ulceration (especially in the obese)
- Skin picking
- Altered temperature sensation
- Decreased saliva flow
- High vomiting threshold
- Seizures (in 10%-20%)
Morbidity and mortality. Mortality rate in PWS is higher than in controls with intellectual disability, with obesity and its complications being factors [Einfeld et al 2006]. Based on a population study, the death rate was estimated at 3% per year [Butler et al 2002], although a later study of the same population showed the rate to be decreasing to 1.25% per annum with improved management [Whittington et al 2015]. Two multicenter series of individuals who died of PWS have been reported [Schrander-Stumpel et al 2004, Stevenson et al 2004], and an extensive case and literature review of 64 cases of death in PWS was performed [Tauber et al 2008]. Respiratory and other febrile illnesses were the most frequent causes of death in children, and obesity-related cardiovascular problems and gastric causes or sleep apnea were most frequent in adults. Other causes of morbidity include diabetes mellitus, thrombophlebitis, and skin problems (e.g., chronic edema, infection from skin picking).
A few individuals have been reported to have respiratory or gastrointestinal infections resulting in unexpected death; of these, three who died as a result were noted to have small adrenal glands [Stevenson et al 2004], although this is not a common finding. The report of central adrenal insufficiency (CAI) in 60% of tested individuals [de Lind van Wijngaarden et al 2008] suggests a possible explanation for some of these unexpected and sudden deaths. Other studies have not demonstrated a high incidence [Farholt et al 2011, Grugni et al 2013] of CAI in PWS.
Acute gastric distention and necrosis have been reported in a number of individuals with PWS [Stevenson et al 2007a], particularly following an eating binge among those who are thin but were previously obese. It may be unrecognized because of high pain threshold and can be fatal.
Choking, especially on hot dogs, has been reported as cause of death in approximately 8% of deaths in individuals with PWS [Stevenson et al 2007b].
Concern about the possible contribution of growth hormone (GH) administration to unexpected death has been raised by reported deaths of individuals within a few months of starting GH therapy [Eiholzer 2005, Sacco & Di Giorgio 2005]. The few reported deaths were mostly in obese individuals who had pre-existing respiratory or cardiac disorders with evidence of upper airway obstruction and uncorrected tonsillar and adenoidal hypertrophy. In the database of one pharmaceutical company, five of 675 children treated with GH died suddenly of respiratory problems [Craig et al 2006]. In another study, the rate of death in affected individuals on and off GH did not differ [Nagai et al 2005]. A study of the natural history of PWS in one region of the UK found the overall death rate of individuals with PWS to be as high as 3% per year without GH therapy [Whittington et al 2001]. Thus, the relationship of GH administration to unexpected death remains unclear. However, it is advisable to obtain a sleep study before the initiation of GH therapy and again four to eight weeks after the beginning of GH therapy to ensure that GH treatment has not caused or worsened sleep-disordered breathing [Miller et al 2006b]. A long-term study of 48 treated children suggests that the benefits of treatment with GH greatly exceed the risks [Carrel et al 2010].
Neuroimaging. In one study, all 20 individuals with PWS who were evaluated had brain abnormalities that were not found in 21 sibs or 16 individuals with early-onset morbid obesity who did not have PWS [Miller et al 2007]. All had ventriculomegaly; 50% had decreased volume of brain tissue in the parietal-occipital lobe; 60% had Sylvan fissure polymicrogyria; and 65% had incomplete insular closure. In another study, these authors reported white matter lesions in some people with PWS [Miller et al 2006a]. A study of brain MRIs from 91 individuals with PWS from another group showed reduced pituitary height in 49% and some neuroradiologic abnormality in 67% [Iughetti et al 2008]. The implications of these findings are unknown.
Genotype-Phenotype Correlations
No phenotypic feature is known to correlate exclusively with any one of the three main molecular mechanisms that result in PWS. However, some statistical differences in the frequency or severity of certain features between the two largest molecular classes (deletion and UPD) are observed.
UPD
- Post-term delivery is more common with UPD [Butler et al 2009].
- Individuals with UPD are less likely to have the typical facial appearance, hypopigmentation, or skill with jigsaw puzzles [Dykens 2002]; they also have a somewhat higher verbal IQ and milder behavior problems [Dykens et al 1999, Roof et al 2000, Hartley et al 2005].
- Individuals with UPD are more likely to have psychosis [Holland et al 2003, Yang et al 2013] and autism spectrum disorders [Veltman et al 2004, Whittington et al 2004b, Veltman et al 2005, Descheemaeker et al 2006]. Studies suggest that as many as 62% of those with UPD develop atypical psychosis compared with 16% of those with a deletion [Soni et al 2007].
Deletion
- Individuals with a deletion showed a higher frequency of need for special feeding techniques, sleep disturbance, hypopigmentation, and speech articulation defects in a recent study of 91 children [Torrado et al 2007].
- Individuals with the slightly larger, type 1 deletions (BP1 to BP3; see Figure 2) have been reported to have more compulsions and poorer adaptive behavior, intellectual ability, and academic achievement than those with type 2 deletions (BP2 to BP3) [Butler et al 2004, Hartley et al 2005]. Two other studies found much less clinically significant differences between individuals with these two deletion types [Milner et al 2005, Varela et al 2005]. Larger studies are needed to determine whether there are significant clinical differences between the two most frequent deletion classes.

Figure 2.
Summary of the genetic and expression map of chromosome region 15q11.2-q13 The Prader-Willi syndrome (PWS) region (shown in blue) has five paternal-only (PWS region) expressed unique-copy genes that encode polypeptides (MKRN3, MAGEL2, NECDIN, and SNURF-SNRPN) (more...)
Penetrance
Penetrance is complete.
Nomenclature
The term HHHO (hypogonadism, hypotonia, hypomentia, obesity) is no longer used.
The condition is sometimes called Willi-Prader syndrome or Prader-Labhart-Willi syndrome.
Prevalence
The estimated prevalence of PWS is 1:10,000 to 1:30,000 in a number of populations.
Differential Diagnosis
Many disorders can mimic parts of the PWS phenotype.
Craniopharyngioma and the results of its treatment show the greatest overlap with PWS. Damage to the hypothalamus causes most of the same findings that characterize PWS, particularly when craniopharygioma occurs at an early age. History and, if uncertain, DNA methylation analysis will distinguish craniopharyngioma from PWS.
Hyperphagic short stature is an acquired condition related to psychosocial stress that includes growth hormone insufficiency, hyperphagia, and mild learning disabilities [Gilmour et al 2001]. History and, if uncertain, DNA methylation analysis should distinguish this disorder from PWS.
Hypotonia in infancy is also seen in the following conditions:
- Neonatal sepsis
- Central nervous system depression
- Congenital myotonic dystrophy type 1, characterized by hypotonia and severe generalized weakness at birth, often with respiratory insufficiency and early death; intellectual disability is common. It is caused by expansion of a CTG trinucleotide repeat in DMPK.
- Several myopathies and neuropathies, including some instances of spinal muscular atrophy (SMA) [Miller et al 1999, Richer et al 2001]. In these situations, poor respiratory effort may be present, a feature rarely seen in PWS. Molecular genetic testing, EMG/NCV, and/or muscle biopsy are often required to differentiate these conditions.
- Angelman syndrome (AS), characterized by severe developmental delay or intellectual disability, severe speech impairment, gait ataxia and/or tremulousness of the limbs, and a unique behavior with an inappropriate happy demeanor that includes frequent laughing, smiling, and excitability. Microcephaly and seizures are also common. AS is caused by deficient expression or function of the maternally inherited UBE3A allele and may be diagnosed in 75%-80% of individuals with AS using DNA methylation analysis of chromosome 15. In infancy, hypotonia may be the only manifestation of AS. Affected individuals lack the characteristic sucking problems, hypogonadism, and facial appearance of individuals with PWS.
- Fragile X syndrome, characterized by moderate intellectual disability in affected males and mild intellectual disability in affected females. Males may have a characteristic appearance (large head, long face, prominent forehead and chin, protruding ears), connective tissue findings (joint laxity), and large testes (postpubertally). Behavioral abnormalities, sometimes including autism spectrum disorder, are common. The diagnosis of fragile X syndrome rests on the detection of an alteration in FMR1 consisting of expansion of a triplet repeat and aberrant gene methylation. In infancy, hypotonia may be the only manifestation. Affected individuals lack the characteristic sucking problems, hypogonadism, and facial appearance of individuals with PWS.
- WAC-related intellectual disability is typically characterized by variable degrees of global developmental delay and/or intellectual disability. Behavioral abnormalities including anxiety, attention deficit/hyperactivity disorder, and/or autism spectrum disorder are observed in the majority of older children and adults. Most affected infants have significant but nonspecific features at birth such as neonatal hypotonia and feeding problems. Some affected individuals come to medical attention with respiratory or vision problems. Facial features may be mildly dysmorphic, but are nonspecific. To date, 18 individuals have been identified with WAC-related ID. The diagnosis of WAC-related ID is established in a proband by identification of a heterozygous pathogenic variant in WAC on molecular genetic testing.
Developmental delay/intellectual disability and obesity with or without hypogonadism can be seen in the following disorders:
- Angelman syndrome (AS). Individuals with AS caused by paternal UPD 15 frequently have an elevated BMI for age. In one large study overweight was found in more than 70% and obesity in more than 40% [Lossie et al 2001].
- Fragile X syndrome. A subset have been found with a "Prader-Willi-like" phenotype including hyperphagia and obesity [de Vries et al 1993].
- Maternal uniparental disomy for chromosome 14, which also includes prenatal growth retardation, feeding problems, short stature, and precocious puberty [Cox et al 2004, Hosoki et al 2009]
- Albright hereditary osteodystrophy (OMIM 103580), which also includes short stature, but lacks hypotonia and has different characteristic facial appearance (round face). Specific testing is possible by measurement of Gs receptor-coupling protein.
- Bardet-Beidl syndrome (BBS), characterized by cone-rod dystrophy, truncal obesity, postaxial polydactyly, cognitive impairment, male hypogonadotropic hypogonadism, complex female genitourinary malformations, and renal dysfunction. Individuals with BBS have a different facial phenotype from those with PWS. Inheritance is typically autosomal recessive, although in fewer than 10% of individuals inheritance may be more complex.
- Cohen syndrome, characterized by failure to thrive in infancy and childhood; truncal obesity in the teen years; early-onset hypotonia and developmental delays; microcephaly developing during the first year of life; moderate to profound psychomotor retardation; progressive retinochoroidal dystrophy and high myopia; neutropenia in many with recurrent infections and aphthous ulcers in some; a cheerful disposition; joint hypermobility; and characteristic facial features which are different from PWS. Cohen syndrome is caused by pathogenic variants in VPS13B. Inheritance is autosomal recessive.
- Borjeson-Forssman-Lehmann syndrome (OMIM 301900), seen in males, characterized by severe cognitive deficit, epilepsy, hypogonadism, hypometabolism, marked obesity, infantile hypotonia and failure to thrive, and short stature. It can be distinguished by the severity of intellectual disability, the presence of nystagmus, and characteristic facial appearance with prominent superciliary ridges, ptosis, and deep-set eyes. Pathogenic variants in PHF6 are causative. Inheritance is X-linked. Heterozygous females who show manifestations of the disorder have skewed X-chromosome inactivation or a genomic deletion including PHG6.
- Alstrom syndrome, characterized by cone-rod dystrophy, early-onset obesity, progressive sensorineural hearing impairment, dilated cardiomyopathy (>60%), the insulin resistance syndrome/type 2 diabetes mellitus associated with acanthosis nigricans, and developmental delay (~50%). Other endocrine abnormalities can include hypothyroidism and male hypogonadotropic hypogonadism. Urologic disorders of varying severity, characterized by detrusor-urethral dyssynergia, appear in females in their late teens. Severe renal disease is usually a late finding. Pathogenic variants in ALMS1 are found in 70%-80% of individuals of northern European descent, and about 40% of affected individuals worldwide.
Cytogenetic abnormalities with a similar phenotype include the following:
- A "PWS-like phenotype" of syndromic obesity has been identified in individuals with an interstitial deletion of 6q16.2, which includes SIM1 [Varela et al 2005]. This deletion had been reported at least five times previously in syndromic obesity [Bonaglia et al 2008] as well a duplication [Desch et al 2015]. Also, pathogenic variants in SIM1 alone have been found in patients with severe obesity [Bonnefond et al 2013, Ramachandrappa et al 2013].
- Several reports have associated a Prader-Willi-like phenotype with 1p36 deletion; additional findings include hypotonia, developmental delay, obesity, hyperphagia, and behavioral problems [Tsuyusaki et al 2010, Stagi et al 2014].
- Multiple reports describe deletions at 16p11.2 including SHB2B1, which is involved in leptin and insulin signaling [Maillard et al 2015].
- Reports of other cytogenetic anomalies in individuals with a PWS-like phenotype have included dupXq27.2-ter and del10q26 [Lukusa & Fryns 2000, Ben-Abdallah-Bouhjar et al 2012, Rocha & Paiva 2014].
Management
Management of the manifestations of Prader-Willi syndrome (PWS) is age dependent and should include both addressing of the consequences of PWS and anticipatory guidance. It is recommended that a team approach be used, if possible. Several approaches to management have been published [Goldstone et al 2008, Cassidy & Driscoll 2009, Cassidy & McCandless 2010, McCandless 2011, Cassidy et al 2012].
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with PWS, the following evaluations are recommended:
- Consultation with a clinical geneticist and/or genetic counselor
- Endocrinology consultation
- Nutritional consultation
- Assessment of newborns and young infants for sucking problems and failure to thrive
- Regardless of age, measurement and plotting of height, weight, head circumference and body mass index (BMI) on either age-appropriate growth charts or charts developed for PWS [Butler et al 2011, Butler et al 2015]
- Assessment for hypothyroidism in children, especially those with prolonged failure to thrive, those with excess weight gain in the absence of increased food intake, and those with poor linear growth despite growth hormone treatment
- Evaluation of respiratory status with a low threshold for performing a sleep study regardless of age. These studies are specifically recommended prior to the initiation of growth hormone therapy (GHT) and four to eight weeks after starting GHT, along with assessment of the size of tonsils and adenoids, particularly in the obese individual.
- Assessment of development of infants and of educational development of children including a speech evaluation
- Assessment for the presence of behavioral problems and obsessive-compulsive features after age two years, and for psychosis in adolescents and adults. If history reveals evidence of these problems, referral for more detailed assessment is indicated.
- Assessment of males for the presence of cryptorchidism regardless of age
- Referral for ophthalmologic evaluation if strabismus is present, and for assessment of visual acuity by age one year or at diagnosis if it is later
- Regardless of age, assessment for the presence of scoliosis clinically, and, if indicated, radiographicallyNote: Very obese individuals cannot be adequately assessed for scoliosis clinically; x-rays are necessary to establish the diagnosis.
Treatment of Manifestations
A team approach to management is recommended [Goldstone et al 2008, Cassidy & McCandless 2010].
Feeding, hyperphagia, and obesity. Special feeding techniques, including special nipples or gavage feeding, may be necessary for the first weeks to months of life to assure adequate nutrition and avoid failure to thrive. Individuals diagnosed with PWS typically do not need a G-tube since the feeding will improve with time.
When weight centiles begin increasing in nutritional phase 2a (typically between 18 and 36 months), a program of a well-balanced, low-calorie diet, regular exercise, and close supervision to minimize food stealing should be instituted to prevent obesity (i.e., BMI Z score of <2) and its consequences. The same program is appropriate if obesity is present at any time. Consultation with a dietician and close follow up are usually necessary, and locking the kitchen, refrigerator, and/or cupboards is often needed once the child is able to open the refrigerator and cupboards. Caloric needs of infants and children with PWS are typically 60%-80% of the recommended daily allowance (RDA). The energy requirement of adults with PWS, which rarely exceeds 1200-1400 Kcal/day, should be considered in planning daily food intake. Assessment of adequacy of vitamin and mineral intake by a dietician, and prescription of appropriate supplementation, is indicated, especially for calcium and vitamin D.
No medications are known to aid in controlling hyperphagia, but several clinical trials are underway (see Therapies Under Investigation).
Gastric bypass is not recommended in PWS, as it does not appear to correct the lack of satiety and will not prevent overeating. In addition, complication rates are high [Scheimann et al 2012].
Growth hormone treatment normalizes height, increases lean body mass, decreases fat mass, and increases mobility, which are beneficial to weight management. Dose recommendations in young children are generally similar to those for individuals with isolated growth hormone deficiency (i.e., ~1 mg/m2/day) but the dose must be individualized as the child grows. Therapy can be started in infancy or at the time of diagnosis. The adult dose of growth hormone is 20%-25% of the dose recommended in children. Monitoring of growth velocity, head circumference, and serum IGF-1 is important to avoid overtreatment.
Controlled trials of growth hormone therapies have demonstrated significant benefit from infancy through adulthood [Carrel et al 2010, Sode-Carlsen et al 2010, Wolfgram et al 2013].
- An increase in language and cognitive skills in treated infants [Myers et al 2007] and an improvement in mental speed and flexibility as well as motor performance in adults [Höybye et al 2005] have been reported based on controlled trials.
- A review of the results of one to two years of growth hormone treatment among 328 children documented in the database of one pharmaceutical company indicated improved height velocity, particularly in prepubertal children, but no change in BMI [Craig et al 2006].
- Significantly greater adult height was demonstrated in 21 individuals treated long term versus 39 untreated individuals without an increase in adverse side effects [Angulo et al 2007].
- Some improvements in cognition have been suggested with growth hormone therapy in individuals with PWS [Osório 2012, Siemensma 2012], but more work and longer studies need to be done.
- Although there was initial concern about growth hormone treatment contributing to scoliosis in PWS, later studies showed no difference in frequency or severity in those treated compared to those who were not treated [Nagai et al 2006, Angulo et al 2007].
Decreased saliva production can be addressed with products developed for the treatment of dry mouth, including special toothpastes, gels, mouthwash, and gum.
Therapies, education and behavior management. Early intervention in children before age three years, particularly physical therapy, may improve muscle strength and encourage achievement of developmental milestones. In older individuals, daily muscle training increases physical activity and lean body mass [Schlumpf et al 2006].
Initiate appropriate educational programming in children:
- Begin speech therapy for language delay and articulation abnormalities in infancy and childhood.
- Special education, either in an inclusion setting or in a self-contained classroom setting, is usually necessary during school age. An individual aide is helpful in assuring attendance to task. Social skills training groups have been beneficial.
Behavioral disturbance should be addressed with behavioral management programs, including firm limit setting. While no medication is beneficial in managing behavior in all individuals with PWS, serotonin reuptake inhibitors have helped the largest proportion of affected teenagers and adults, particularly those with obsessive-compulsive symptoms [Brice 2000, Dykens & Shah 2003].
Psychosis is reported to respond well to selective serotonin reuptake inhibitors, but not to mood stabilizers [Soni et al 2007]. There are no well-designed studies of the effectiveness of treatment for psychosis in PWS [Ho & Dimitropoulos 2010].
Hypogonadism. Cryptorchidism may resolve spontaneously, even up to adolescence, but usually requires hormonal and surgical approaches; however, preservation of fertility is not an issue. Standard treatment is appropriate. Human chorionic gonadotropin treatment for infants with undescended testes should be considered as it can improve the size of the scrotal sac and improve surgical outcome [McCandless 2011; Angulo & Miller, unpublished data].
Replacement of sex hormones produces adequate secondary sexual characteristics but is somewhat controversial because of the possible role of testosterone replacement in behavior problems in males and the role of estrogen replacement in the risk of stroke as well as hygiene concerns related to menstruation in females. Daily use of the testosterone patch or gel may avert exacerbation of behavioral problems by providing a more even blood level than use of a slow-release depo-testosterone injection every month. Also, it was shown in the non-PWS adult population that depo- testosterone injections were associated with a greater risk of cardiovascular events, hospitalizations, and deaths compared with gels and patches [Layton et al 2015].
Concern about osteoporosis should be considered in deciding about hormone replacement. Recent reports of fertility in four women with PWS raise the issue of need for birth control [Akefeldt et al 1999; Schulze et al 2001; Vats & Cassidy, unpublished data].
Sleep issues. Disturbed sleep in children and adults should prompt a sleep study, as treatment may be available. Treatment depends on the cause and may include tonsillectomy and adenoidectomy and/or CPAP or BiPAP, as in the general population.
Excessive daytime sleepiness (unrelated to the degree of sleep apnea) is frequently seen in individuals with PWS. Modafinil has been shown to be a safe and effective treatment for this condition [De Cock et al 2011].
Skin picking. One study demonstrated decreased skin picking with topiramate treatment in some individuals [Shapira et al 2004]; other clinicians have reported anecdotally that approximately half of individuals with PWS who skin pick benefit from low-dose (25-50 mg daily) topiramate.
A recent study of 35 individuals with PWS (age 5-39 years) using 450-1200 mg/day of N-acetylcysteine found a high degree of success in reducing or eliminating skin picking [Miller & Angulo 2014].
Other
- Management of strabismus is as for any infant.
- Management of scoliosis, hip dysplasia, and complications of obesity is as in the general population.
Adulthood. For adults with PWS, the most successful living situation for behavior and weight management is a group home specially designated for individuals with PWS, where diet and access to food are tightly restricted and exercise is included in daily activities. Affected individuals generally require a sheltered employment environment.
Issues of guardianship, wills, trusts, and advocacy should be investigated no later than adolescence.
Prevention of Primary Manifestations
Obesity may be prevented if the diet, exercise, and supervision program described in Treatment of Manifestations is instituted. Early diagnosis allows the clinician to begin anticipatory guidance concerning the natural history of PWS, and in particular the nutritional phases, informing the family about the risk of obesity and the need to monitor weight increase and to restrict calories beginning around 18-36 months of age.
If started at a young age, growth hormone treatment, along with good dietary control, may retard obesity and the high proportion of fat mass. It may also prevent development of the typical facial appearance and improve motor milestones.
Prevention of Secondary Complications
Diabetes mellitus rarely occurs in the absence of obesity.
Calcium and vitamin D supplementation are probably beneficial, as low-calorie diets are often low in dairy products and osteoporosis has been documented in the majority of older children and adults with PWS.
If osteoporosis develops, consider treatment with a bisphosphonate.
Although no formal study exists, individuals with PWS tend to be very sensitive to medications of all kinds. Starting with lower doses is recommended.
Surveillance
Health supervision guidelines from the American Academy of Pediatrics (AAP) have been published [McCandless 2011] (full text).
To assure appropriateness of exercise program and diet, including adequacy of vitamin and mineral intake and monitor height, weight, and BMI (weight in kg/height in m2):
- Every month in infancy;
- Every six months in the first decade of life;
- At least annually thereafter.
Cryptorchidism can recur after orchidopexy; therefore, testicular position should be monitored.
Evaluate for the presence of diabetes mellitus by standard methods (e.g., obtaining glycosylated hemoglobin concentration and/or glucose tolerance test) in anyone with significant obesity or rapid significant weight gain.
Test annually for hypothyroidism, including free T4 and TSH levels.
Obtain history of any sleep disturbance; if present, obtain a sleep study.
Monitor for development of scoliosis clinically or, in the presence of obesity, radiographically at least annually.
Perform bone densitometry by DEXA to evaluate for possible osteoporosis every two years in adulthood.
Obtain history for behavioral and psychiatric disturbance at least annually.
Evaluation of Relatives at Risk
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Therapies Under Investigation
In the last few years there has been great interest by the pharmaceutical industry in testing treatments for the major manifestations of PWS – particularly the hyperphagia, obesity, and behavioral problems. For a detailed description of these studies, click here (pdf).
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.