Wiskott-Aldrich Syndrome
A number sign (#) is used with this entry because Wiskott-Aldrich syndrome (WAS) is caused by mutation in the WAS gene (300392) on chromosome Xp11.
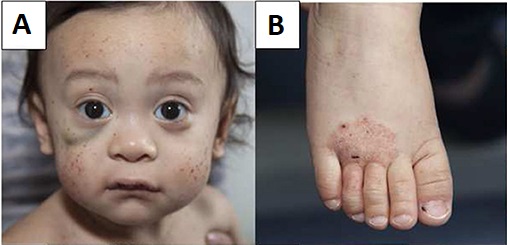
DescriptionWiskott-Aldrich syndrome (WAS) is an X-linked recessive immunodeficiency characterized by thrombocytopenia, eczema, and recurrent infections (Lemahieu et al., 1999).
Genetic Heterogeneity of Wiskott-Aldrich Syndrome
See Wiskott-Aldrich syndrome-2 (WAS2; 614493), caused by mutation in the WIPF1 gene (602357). Also see 600903 for a possible autosomal dominant form of the disorder.
Clinical FeaturesThe manifestations of Wiskott-Aldrich syndrome are eczema, thrombocytopenia, proneness to infection, and bloody diarrhea. Death usually occurs before age 10 years. The original American kindred reported by Aldrich et al. (1954) was of Dutch extraction; the 3 patients of Wiskott (1937) were German. Wiskott, who worked in Munich, referred to the disorder in his patients as 'Werlhof's disease,' the eponymic designation for thrombocytopenic purpura. Van den Bosch and Drukker (1964) described several families in the Netherlands. In 3 of 5 female carriers, the platelet count was below the lower limit of normal.
Perry et al. (1980) reported that median survival increased from 8 months for patients born before 1935 to 6.5 years for those born after 1964. One patient had survived to age 36 years at the time of the survey. Causes of death were mainly infections or bleeding, but 36 of the 301 patients (12%) developed malignancies: lymphoreticular tumors in 23 and leukemia in 7. Ten Bensel et al. (1966) called attention to the occurrence of malignancy of the reticuloendothelial system, which they saw in 2 of 4 sibs and found in 5 reported cases.
Capsoni et al. (1986) described a 19-year-old man with WAS. Only 7 affected persons over age 18 had been described previously. Standen et al. (1986) reported a kindred with 13 males in 6 sibships, related through females, with inherited thrombocytopenia thought to be a variant of WAS because it was associated with elevated serum IgA and mild nephropathy. Five suffered from severe eczema since infancy but had no unusual susceptibility to infections. Platelet volume was reduced. Gutenberger et al. (1970) reported a similar family. Renal biopsy was performed in 3 patients. In the first, advanced membranoproliferative glomerulonephritis was found with deposition of complement and IgG on the basement membrane. In the second, mesangial glomerulonephritis with focal glomerulosclerosis and deposition of complement and IgA were found. The third showed minimal glomerulonephritis. Standen et al. (1986) concluded that despite the clinical similarities and the elevated IgA in both conditions, the disorder is distinct from Berger disease (161950). Spitler et al. (1980) found nephropathy in 5 of 32 patients with WAS who participated in a study of treatment with transfer factor, a dialyzable extract of leukocytes that enhances cellular immunity. Although nephropathy occurred without such treatment, the temporal relationships suggested that transfer factor aggravated the problem.
McEnery and Nash (1973) described 2 unrelated males with the association of WAS and infantile cortical hyperostosis (Caffey disease; 114000), and Abinun et al. (1988) also described a case. Thus, an immunologic defect may play a role in the pathogenesis of infantile cortical hyperostosis. Meropol et al. (1992) reported the case of a 24-year-old man with WAS complicated by T-cell large cell lymphoma and Kaposi sarcoma (148000). Kaposi sarcoma is well known in connection with the immunosuppression used with allograft transplantation and in patients with HIV infection, but this was the first incidence of its occurrence in this form of immunodeficiency.
Sullivan et al. (1994) reported on a multiinstitutional survey of WAS in the U.S. in which laboratory and clinical data were collected on 154 affected individuals. There was a family history of the disorder in the case of 74 of the patients. Thrombocytopenia was a prerequisite for entry into the study; however, only 27% of patients had the typical set of 3 symptoms described originally by Aldrich et al. (1954). The immunologic findings in particular varied considerably with the most distinctive finding: that 61% of the patients had a low CD8+ count. Eczema developed in 81% but was not always present at diagnosis. In those patients in whom platelet size was measured, Sullivan et al. (1994) found them to be small, although they did increase in size following splenectomy. The average age at diagnosis was 21 months; the average age at death was 8 years. There were 16 patients who lived beyond 18 years, and the prognosis for the disorder had improved considerably in recent years. Bone marrow transplantation had been carried out in 47 cases and a good outcome was reported in two-thirds of them. Autoimmune disorders occurred in 40% of patients; this group had a poor prognosis as they were more likely to develop a malignancy. Malignancies were seen in 13% of patients and were mainly of the lymphoreticular system.
Du et al. (2006) described somatic mosaicism in a 15-year-old male WAS patient due to a second-hit mutation in the initiation codon. See 300392.0019-300392.0020. The patient had no clear family history. Thrombocytopenia was noticed at 1 month of age and thereafter eczema and recurrent infections were clinical features. At 8 years of age, he had persistent cough due to pulmonary hilar lymph node swelling. From the result of hilar lymph node biopsy, he was diagnosed with Hodgkin disease and received chemotherapy and local radiotherapy (Sasahara et al., 2001; Sasahara et al., 2002). The patient had remained in complete remission thereafter. His platelet count was in the range of 6,000-15,000/microliter. Episodes of respiratory infections occurred less frequently, although severe eczema and thrombocytopenia persisted.
DiagnosisIn an obligate heterozygote who was heterozygous for the AB polymorphism of G6PD, Gealy et al. (1980) found that only the B isoenzyme was present in platelets and T lymphocytes, although both were present in erythrocytes and neutrophils. Prchal et al. (1980) pursued the implications of this finding for genetic counseling. Although G6PD is likely to be useful in only a limited number of potential carriers, the large number of X-chromosome markers, DNA polymorphisms and other markers now available make it likely that carrier detection will be possible. Shapiro et al. (1978) concluded that carriers can be identified by study of platelets, which show a defect in oxidative phosphorylation.
Fearon et al. (1988) studied the pattern of X-chromosome inactivation in various cell populations from female relatives of patients with WAS, through analysis of the methylation patterns of X-linked genes that display RFLPs. They found that carriers could be accurately identified by the fact that peripheral blood T cells, granulocytes, and B cells of obligate heterozygotes display specific patterns of X-chromosome inactivation that are clearly different from those of normal controls.
Puck et al. (1990) pointed out that the diagnosis of WAS may be difficult in infancy when sporadic thrombocytopenia with no, or only questionable, immunologic abnormalities are present. In the case of 2 unrelated males with this problem, X-chromosome inactivation in the T cells of the mothers showed each of them to have a highly skewed X-chromosome inactivation pattern typical of WAS carriers. In one of the patients, a T-cell defect was subsequently demonstrated directly by studies of the lymphocytes, which failed to proliferate in periodate and anti-CD43. Notarangelo et al. (1991) reported a similar case of a boy with WAS presenting as idiopathic thrombocytopenia.
Notarangelo et al. (1991) studied a presumably heterozygous, thrombocytopenic female from a WAS pedigree. Her carrier status was confirmed by linkage studies. Both small-sized and normal-sized platelets were present, suggesting that, unlike the vast majority of WAS carriers, she did not manifest nonrandom X-chromosome inactivation in the thrombopoietic cell lineage. Studies of X-chromosome inactivation by means of RFLP and methylation analysis showed that the pattern of X-chromosome inactivation was nonrandom in T lymphocytes but random in granulocytes. Notarangelo et al. (1993) reviewed the use of the biased inactivation of the X chromosome in hematopoietic cells as a tool for carrier detection in connection with genetic counseling. A closely linked hypervariable marker, M27-beta (DXS255), was used.
Yamada et al. (1999) showed that flow cytometric analysis of WASP expression in lymphocytes is useful in the diagnosis of WAS. They found that intracellular WASP is expressed as distinctly 'bright' and 'dim' phenotypes in lymphocytes from normal individuals and WAS patients, respectively. Yamada et al. (2000) demonstrated that WAS carriers could also be identified by flow cytometric analysis of monocytes but not lymphocytes. Bright and dim phenotypes for normal individuals and patients, respectively, were observed in monocytes, whereas in carriers, mixed populations (to varying degrees) of bright- and dim-staining cells were detected. The authors noted that flow cytometry is a simpler and more rapid method of diagnosis than molecular methods but may not be sensitive enough to detect carriers with low percentages of WASP-dim monocytes.
Prenatal Diagnosis
Holmberg et al. (1983) found that normal midtrimester fetuses have platelets of the same size as normal newborns and adults. They used these data 'to exclude Wiskott-Aldrich syndrome in an 18-week fetus at 50% risk of being affected.' Unfortunately, we do not know that the platelets of the WAS fetus are abnormally small.
Schwartz et al. (1989) described the first-trimester diagnosis and exclusion of WAS by means of closely linked DNA markers.
In 2 unrelated families, Giliani et al. (1999) performed successful prenatal diagnosis of WAS at week 12 of gestation, using a combined nonradioactive analysis of SSCP and heteroduplex formation, followed by automated sequencing.
Clinical ManagementCorash et al. (1985) studied the mechanism of the usual improvement in thrombocytopenia in WAS after splenectomy. The thrombocytopenia is accompanied by elevated platelet-associated IgG and low mean platelet size. Both return to normal after splenectomy. Patients who relapse redevelop elevated IgG but maintain normal platelet size.
Webb et al. (1993) described their experience with renal transplantation in a 46-year-old man with the syndrome of thrombocytopenia with raised IgA levels and impaired renal function. The man had a strong family history of hereditary thrombocytopenia and had presented in early childhood with allergic eczema, asthma, thrombocytopenic purpura, and recurrent middle ear infections. He had a normal platelet count after splenectomy was performed at the age of about 30. In his mid-thirties, he had subtotal colectomy and ileostomy for severe ulcerative colitis. This disorder later recurred, associated with keratitis and arthritis of large joints. He was later admitted to the hospital with a febrile illness, biopsy-proven cutaneous vasculitis, raised IgA levels, and impaired renal function. Renal biopsy demonstrated mesangioproliferative glomerulonephritis, old crescents, and mesangial IgA deposition. After renal transplant, a 'reduced immunosuppressive protocol' was instituted because of his underlying immunologic disorder. Despite this, no rejection episodes occurred.
The first reports of successful bone marrow transplantation for severe combined immunodeficiency (XSCID; 300400) and for WAS were provided by Gatti et al. (1968) and Bach et al. (1968). Fischer et al. (1986) gave a retrospective analysis of results in 162 patients who had undergone transplantation in 14 European centers between 1969 and 1985. Brochstein et al. (1991) reported on the bone marrow transplantation in 17 patients with WAS.
Boztug et al. (2010) reported successful treatment of 2 patients with Wiskott-Aldrich syndrome with transfusion of autologous, genetically modified hematopoietic stem cells. They found sustained expression of WAS protein expression in hematopoietic stem cells, lymphoid and myeloid cells, and platelets after gene therapy. T and B cells, natural killer cells, and monocytes were functionally corrected. After treatment, the patients' clinical condition markedly improved, with resolution of hemorrhagic diathesis, eczema, autoimmunity, and predisposition to severe infection. Comprehensive insertion-site analysis showed vector integration that targeted multiple genes controlling growth and immunologic responses in a persistently polyclonal hematopoiesis that was followed for 3 years in both boys.
Aiuti et al. (2013) reported 3 patients with Wiskott-Aldrich syndrome treated with lentiviral gene-corrected hematopoietic stem cells (HSCs) after pretreatment with a reduced-intensity myeloablative regimen. Administration of autologous HSCs transduced with lentivirus at high efficiency (greater than 90%) resulted in robust (25 to 50%), stable, and long-term engraftment of gene-corrected HSCs in the patients' bone marrow. In all 3 patients, Aiuti et al. (2013) observed improved platelet counts, protection from bleeding and severe infections, and resolution of eczema. In contrast to gamma-retroviral gene therapy, lentiviral-based therapy did not induce in vivo selection of clones carrying integrations near oncogenes. Consistent with this, Aiuti et al. (2013) did not see evidence of clonal expansions in the patients for up to 20 to 32 months after gene therapy.
Population GeneticsPerry et al. (1980) found an incidence of 4.0 per million live male births in the United States.
PathogenesisSeveral groups (Blaese et al., 1968; Cooper et al., 1968) presented evidence that the immune defect is in the afferent limb, i.e., is one of antigen processing or recognition. In an obligate heterozygote who was heterozygous for the AB polymorphism of G6PD (305900), Gealy et al. (1980) found that only the B isoenzyme was present in platelets and T lymphocytes, although both were present in erythrocytes and neutrophils. The findings suggested selection against the WAS gene in these tissues, which are also the ones that express the defect in the hemizygous affected male.
Parkman et al. (1981) studied the surface proteins of lymphocytes and platelets by radioiodination followed by SDS-polyacrylamide gel electrophoresis and autoradiography. All 3 WAS patients studied showed, in lymphocytes, absence of a protein, molecular weight 115,000, found in normals. Platelets also showed an abnormality of surface glycoproteins. CD43 (182160), or sialophorin, is a cell-surface sialoglycoprotein that is deficient in quantity and/or is defective in lymphocytes of patients with this disorder (Parkman et al., 1981; Remold-O'Donnell et al., 1984). Mentzer et al. (1987) suggested that sialophorin functions in T-cell activation.
Simon et al. (1992) presented experimental results indicating the association of WAS with a defect in the coupling of surface immunoglobulin (sIg) on B cells to signal transduction pathways considered prerequisite for B-cell activation, probably at the level of tyrosine phosphorylation.
Symons et al. (1996) proposed that the Wiskott-Aldrich protein provides a link between CDC42 and the actin cytoskeleton. T lymphocytes of affected males with WAS exhibit a severe disturbance of the actin cytoskeleton, suggesting that the WAS protein may regulate its organization. Kolluri et al. (1996) showed that WAS protein interacts with Cdc42, a member of the RHO family of GTPases. This interaction, which is GTP-dependent, was detected in cell lysates, in transient transfections, and with purified recombinant proteins. Different mutant WAS proteins from 3 unrelated affected males retained their ability to interact with Cdc42 but the level of expression of the WAS protein in these mutants was only 2 to 5% of normal. Taken together, these data suggested to Kolluri et al. (1996) that the WAS protein may function as a signal transduction adaptor downstream of Cdc42, and that, in affected males, the cytoskeletal abnormalities may result from a defect in Cdc42 signaling.
MappingPeacocke and Siminovitch (1987) studied 10 kindreds for linkage with RFLPs. Significant linkage was found between WAS and 2 loci, DXS14 and DXS7, that mapped to the proximal short arm of the X chromosome. Maximal lod scores were 4.29 (at theta = 0.03) and 4.12 (at theta = 0.00), respectively. Arveiler et al. (1987) found a strong suggestion of linkage between IMD2 and DXS1, which is located in Xq11-q12. Kwan et al. (1988) concluded from linkage studies that the WAS gene lies between DXS7 (Xp11.3) and DXS14 (Xp11); the likelihood of this position was at least 128 times higher than that of any other interval studied. In a study of 12 WAS families, Kwan et al. (1989) demonstrated linkage to another DNA marker, DXS255, located at Xp11.22; peak lod score = 4.65 at theta = 0.05. Greer et al. (1989) showed linkage between WAS and DXZ1 (lod score = 7.08 at theta = 0.03) and between WAS and the TIMP (305370) locus (lod score = 5.09 at theta = 0.0). Greer et al. (1990) extended the linkage studies, demonstrating strongest linkage (maximum lod score = 10.19 at theta = 0.0) between WAS and the hypervariable DXS255 locus, a marker already mapped between DXS7 and DXS14. De Saint Basile et al. (1989) found close linkage of WAS to DXS255 (maximum lod = 5.42 at theta = 0.00). Kwan et al. (1991) likewise concluded that DXS255 is the closest marker identified; WAS showed a multipoint maximum lod score of 8.59 at 1.2 cM distal to DXS255. Furthermore, they concluded that the TIMP gene must lie distal to WAS; thus, WAS was thought to lie between DXS255 (Xp11.22) and TIMP (Xp11.3). Greer et al. (1992) demonstrated close linkage between the WAS and OATL1 (311240) loci; maximum lod = 6.08 at theta = 0.00. The finding localized the TIMP, OATL1, and WAS loci distal to DXS146 and the OATL1 and WAS loci proximal to TIMP.
Arveiler et al. (1990) showed that failure to demonstrate linkage of WAS to markers known from other families to be closely situated was attributable to germ cell mosaicism in the grandfather of affected males. The same phenomenon has been described in X-linked agammaglobulinemia; see 300300.
De Saint-Basile et al. (1991) studied a family in which 4 members had X-linked thrombocytopenia. Linkage studies showed mapping to the same region of the X chromosome as that found in WAS. Although polymorphonuclear leukocytes showed a normal pattern of X-inactivation, a skewed pattern was demonstrated in lymphocytes. De Saint-Basile et al. (1991) concluded that this was consistent with allelic mutations at the same locus, with the severity of disease varying according to the distinct patterns of hematopoietic cell involvement in obligate carriers.
Kwan et al. (1995) isolated and characterized a polymorphic CA dinucleotide repeat, DXS6940, that lies within 30 kb of the WAS gene.
Molecular GeneticsDerry et al. (1994) found that the WAS gene was not expressed in 2 unrelated patients with Wiskott-Aldrich syndrome, 1 of whom had a single base deletion that produced a frameshift and premature termination of translation (300392.0001). Two additional patients were identified with point mutations that changed the same arginine residue to either a histidine or a leucine (300392.0002-300392.0003).
Villa et al. (1995) presented proof that mutations in the WAS gene can result in X-linked thrombocytopenia characterized by thrombocytopenia with small-sized platelets as an isolated finding (313900). Why some mutations impair only the megakaryocytic lineage and have no apparent effect on the lymphoid lineage was unclear. In a study of 16 WAS patients and 4 X-linked thrombocytopenia patients, Thompson et al. (1999) identified 14 distinct mutations, including 7 novel gene defects.
In an affected grandson of a female first cousin of the 3 patients described originally by Wiskott (1937), Binder et al. (2006) found a 2-nucleotide deletion in exon 1 of the WAS gene (300392.0021).
Dobbs et al. (2007) identified 2 different but contiguous single basepair deletions in maternal cousins with WAS (300392.0022 and 300392.0023, respectively). Their maternal grandmother was found to be a mosaic for the deletions, both of which occurred on the haplotype from the unaffected maternal great-grandfather, consistent with a bichromatid mutation in a male gamete.
Genotype/Phenotype CorrelationsSchindelhauer et al. (1996) found no genotype/phenotype correlation emerge after a comparison of the identified mutations with the resulting clinical picture for a classical WAS phenotype. A mild course, reminiscent of X-linked thrombocytopenia, or an attenuated phenotype was more often associated with missense than with the other types of mutations.
Greer et al. (1996) examined the genotypes and phenotypes of 24 patients with WAS and compared them with other known mutations of the WASP gene. They demonstrated clustering of WASP mutations within the 4 most N-terminal exons of the gene and identified arg86 as the most prominent hotspot for WASP mutations. They noted the prominence of missense mutations among patients with milder forms of WAS, while noting that missense mutations also comprise a substantial portion of mutations in patients with severe forms of the disease. Greer et al. (1996) concluded that phenotypes and genotypes of WAS are not well correlated; phenotypic outcome cannot be reliably predicted on the basis of WASP genotype.
Lemahieu et al. (1999) identified 17 WASP gene mutations, 12 of which were novel. All missense mutations were located in exons 1 to 4. Most of the nonsense, frameshift, and splice site mutations were found in exons 6 to 11. Mutations that alter splice sites led to the synthesis of several types of mRNAs, a fraction of which represented the normally spliced product. The presence of normally spliced transcripts was correlated with a milder phenotype. When one such case was studied by Western blot analysis, reduced amounts of normal-sized WASP were present. In other cases as well, a correlation was found between the amount of normal or mutant WASP present and the phenotypes of the affected individuals. No protein was detected in 2 individuals with severe Wiskott-Aldrich syndrome. Reduced levels of a normal-sized WASP with a missense mutation were seen in 2 individuals with X-linked thrombocytopenia. Lemahieu et al. (1999) concluded that mutation analysis at the DNA level is not sufficient for predicting clinical course, and that studies at the transcript and protein levels are needed for a better assessment.
Wada et al. (2001) provided evidence that in vivo reversion had occurred in the WAS gene in a patient with Wiskott-Aldrich syndrome, resulting in somatic mosaicism. The mutation was a 6-bp insertion (ACGAGG; 300392.0013) which abrogated expression of the WAS protein. Most of the patient's T lymphocytes expressed nearly normal levels of WAS protein. These lymphocytes were found to lack the deleterious mutation and showed a selective growth advantage in vivo. Analysis of the sequence surrounding the mutation site showed that the 6-bp insertion followed a tandem repeat of the same 6 nucleotides. These findings strongly suggested that DNA polymerase slippage was the cause of the original germline insertion mutation in this family and that the same mechanism was responsible for its deletion in one of the proband's T-cell progenitors, thus leading to reversion mosaicism.
That some mutations in WASP result in X-linked thrombocytopenia without the associated features of the Wiskott-Aldrich syndrome is well established. Devriendt et al. (2001) demonstrated, furthermore, that a constitutively activating mutation in WASP can cause X-linked severe congenital neutropenia (SCNX; 300299). See 300392.0012 for the L270P mutation in WASP demonstrated by Devriendt et al. (2001).
Wada et al. (2004) described 2 additional patients from the same family of the man with revertant T-cell lymphocytes reported by Wada et al. (2001). Somatic mosaicism was demonstrated in leukocytes from the first patient that were cryopreserved when he was 22 years old, 11 years before his death from kidney failure. The second patient, 16 years old at the time of report, had a moderate clinical phenotype and developed revertant cells after the age of 14 years. T lymphocytes showed selective in vivo advantage. These results supported DNA polymerase slippage as a common underlying mechanism and indicated that T-cell mosaicism may have different clinical effects in WAS. Wada et al. (2004) stated that sibs with revertant mosaicism had previously been reported (Wada et al., 2003; Waisfisz et al., 1999), but 3 patients with revertant disease in a single kindred was unprecedented.
Boztug et al. (2008) reported 2 Ukrainian brothers, aged 3 and 4 years, respectively, with WAS due to somatic mosaicism for a truncation mutation and multiple different second-site mutations. Flow cytometric analysis of peripheral blood cells showed that each patient had WAS-negative cells resulting from the truncation mutation and a subset of WAS-positive cells that expressed second-site missense WAS mutations. The second-site mutations resulted in the production of altered, but possibly functional, protein. All second-site mutations in both patients occurred in the same nucleotide triplet in which the truncation mutation occurred. Over time, both boys had a decrease in bleeding diathesis and eczema, and normalization of platelet counts. Boztug et al. (2008) suggested that the second-site mutations may confer a proliferative advantage to the affected cells in these patients.
X-Inactivation Status
Wengler et al. (1995) stated that obligate female carriers of the gene for X-linked agammaglobulinemia (300300) show nonrandom X-chromosome inactivation only in B lymphocytes, and obligate female carriers of the gene for X-linked severe combined immunodeficiency (XSCID) show nonrandom X-chromosome inactivation in both T and B lymphocytes, as well as natural killer cells. However, all formed elements of the blood appear to be affected, as a rule, in obligate carriers of WAS, as judged by the criteria of nonrandom X-chromosome inactivation and segregation of G6PD alleles in informative females. Wengler et al. (1995) demonstrated that CD34+ hematopoietic progenitor cells collected from obligate carriers of WAS by apheresis showed nonrandom inactivation. They used PCR analysis of a polymorphic VNTR within the X-linked androgen receptor gene (313700) to demonstrate nonrandom inactivation which clearly must occur early during hematopoietic differentiation.
Parolini et al. (1998) reported X-linked WAS in an 8-year-old girl. She had a sporadic mutation, glu133 to lys, on the paternally derived X chromosome, but had nonrandom X inactivation of the maternal X chromosome in both blood and buccal mucosa. Her mother and maternal grandmother also had nonrandom X inactivation, which suggested to the authors the possibility of a defect in XIST (314670) or some other gene involved in the X-inactivation process. Puck and Willard (1998) commented on the subject of X inactivation in females with X-linked disease in reference to the paper by Parolini et al. (1998).
Lutskiy et al. (2002) described a female heterozygote for a splice site mutation (300392.0017) who presented at 14 months of age with features of WAS (thrombocytopenia, small platelets, and immunologic dysfunction) and had random inactivation of the X chromosome. She appeared to have a defect in the mechanisms that, in disease-free WAS carriers, lead to preferential survival/proliferation of cells bearing the active wildtype X chromosome.
Animal ModelDerry et al. (1995) stated that Wasp may be a candidate for involvement in 'scurfy,' a T cell-mediated fatal lymphoreticular disease of mice that had previously been proposed as a mouse homolog of Wiskott-Aldrich syndrome (Lyon et al., 1990). Northern analysis of sf tissue samples indicated the presence of Wasp mRNA in liver and skin, presumably as a consequence of lymphocyte infiltration, but no abnormalities in the amount or size of mRNA were identified.
HistoryPuck and Candotti (2006) reviewed lessons from the Wiskott-Aldrich syndrome. Alfred Wiskott (1898-1978) was a German authority on childhood pneumonias who reported 3 affected brothers in 1937. In 1954, Robert Aldrich (1917-1998) and colleagues published an independent description of a large Dutch kindred in which segregation analysis showed X-linked recessive inheritance (Aldrich et al., 1954). By 2006, more than 160 different WAS mutations spanning all 12 exons of the gene had been found in more than 270 unrelated families and functional domains had been defined. Binder et al. (2006) described an affected member from the family reported by Wiskott (1937) and defined the specific mutation (300392.0021). The patient studied was a first cousin twice removed of the originally reported brothers. In a span of 2 generations, a fatal condition had become treatable. The patient had been successfully cured by transplantation by bone marrow from a matched, unrelated donor.