Xeroderma Pigmentosum
Summary
Clinical characteristics.
Xeroderma pigmentosum (XP) is characterized by:
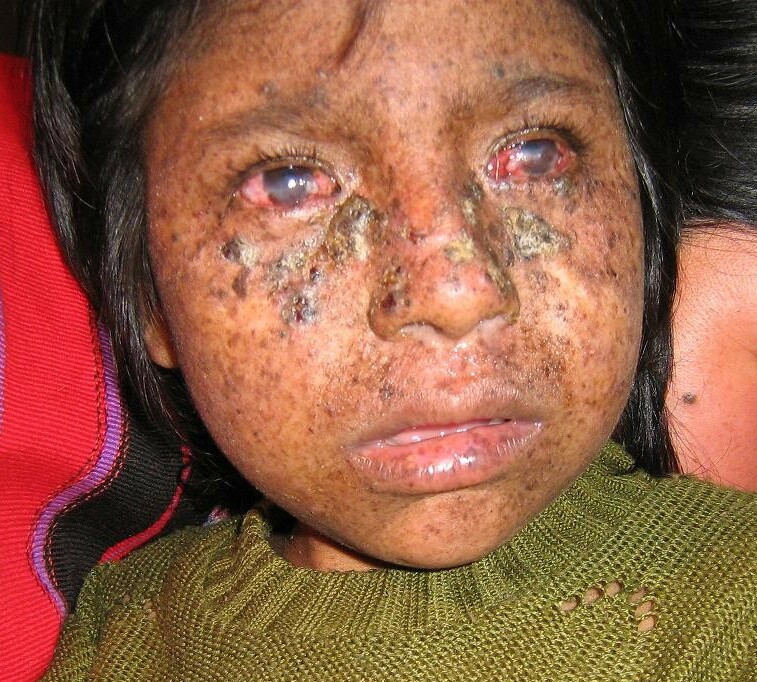
- Sun sensitivity (severe sunburn with blistering, persistent erythema on minimal sun exposure in ~60% of affected individuals), with marked freckle-like pigmentation of the face before age two years in most affected individuals;
- Sunlight-induced ocular involvement (photophobia, keratitis, atrophy of the skin of the lids);
- Greatly increased risk of sunlight-induced cutaneous neoplasms (basal cell carcinoma, squamous cell carcinoma, melanoma).
Approximately 25% of affected individuals have neurologic manifestations (acquired microcephaly, diminished or absent deep tendon stretch reflexes, progressive sensorineural hearing loss, and progressive cognitive impairment). The most common causes of death are skin cancer, neurologic degeneration, and internal cancer. The median age at death in persons with XP with neurodegeneration (29 years) was found to be younger than that in persons with XP without neurodegeneration (37 years).
Diagnosis/testing.
The diagnosis of XP is made on the basis of clinical findings and family history and/or by the identification of biallelic pathogenic variants in DDB2, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, POLH, XPA, or XPC.
Management.
Treatment of manifestations: Small, premalignant skin lesions such as actinic keratoses can be treated by freezing with liquid nitrogen; larger areas can be treated with field treatments such as topical 5-fluorouracil or imiquimod. Rarely, therapeutic dermatome shaving or dermabrasion has been used; skin neoplasms can be treated (as in persons without XP) with electrodesiccation and curettage, or surgical excision; skin cancers that are recurrent or in locations at high risk for recurrence are best treated with Mohs micrographic surgery. Oral isotretinoin or acitretin can prevent new skin neoplasms but have many side effects. Neoplasms of the eyelids, conjunctiva, and cornea can be treated surgically; corneal transplantation may improve the visual impairment resulting from severe keratitis. Hearing loss may be treated with hearing aids.
Prevention of primary manifestations: Avoid sun and other UV exposure to the skin and eyes; measurement of UV light with a light meter in an affected individual's home, school, and/or work environment so that high levels of environmental UV can be identified and eliminated.
Prevention of secondary complications: Dietary supplementation with oral vitamin D as needed.
Surveillance: Skin examinations by a physician every three to 12 months; periodic routine eye and neurologic examinations and audiograms.
Agents/circumstances to avoid: UV exposure from sunlight and artificial sources of UV radiation, cigarette smoke.
Evaluation of relatives at risk: If family-specific pathogenic variants have been identified, molecular genetic testing of at-risk sibs can permit early diagnosis and rigorous sun protection from an early age.
Pregnancy management: Systemic retinoids (isotretinoin, acitretin) may be used as skin cancer chemopreventive agents. These drugs are known to be teratogenic to a developing fetus and pose a high risk for birth defects. Women of reproductive age who are taking a systemic retinoid must use effective contraception and be monitored with regular pregnancy tests.
Genetic counseling.
XP is inherited in an autosomal recessive manner. At conception, each sib of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier. Once the pathogenic variants have been identified in an affected family member, carrier testing for at-risk family members and prenatal testing for pregnancies at increased risk are possible.
Diagnosis
Suggestive Findings
Xeroderma pigmentosum (XP) should be suspected in individuals with the following skin, eye, nervous system, and family history findings.
Skin
- Acute sun sensitivity (severe sunburn with blistering or persistent erythema on minimal sun exposure)
- Marked freckle-like pigmentation (lentigos) on the face before age two years
- Skin cancer within the first decade of life
Eye
- Photophobia with prominent conjunctival injection
- Severe keratitis, sometimes resulting in corneal opacification and vascularization
- Increased pigmentation of the lids with loss of lashes
- Atrophy of the skin of the lids resulting in ectropion, entropion, or in severe cases, complete loss of the lids
Nervous system
- Diminished or absent deep tendon stretch reflexes. EMG and nerve conduction velocities may show an axonal (or mixed) neuropathy.
- Progressive sensorineural hearing loss. Audiometry may reveal early high-tone hearing loss.
- Acquired microcephaly. CT and MRI of the brain may show enlarged ventricles with thinning of the cortex and thickening of the bones of the skull.
- Progressive cognitive impairment
Family history
- Consistent with autosomal recessive inheritanceNote: Absence of a family history of XP does not preclude the diagnosis.
Establishing the Diagnosis
The diagnosis of XP is established in a proband on the basis of clinical findings and family history (see Suggestive Findings) and/or by the identification of biallelic pathogenic variants in one of the genes listed in Table 1.
Molecular testing approaches can include serial single-gene testing, use of a multigene panel, and more comprehensive genomic testing.
Serial single gene testing. The choice of which genes to analyze can be guided by the clinical features and the relative frequency in the population where the individual was born (see Table 1, Table 2, Figure 1, and DiGiovanna & Kraemer [2012]).

Figure 1.
Relationship between genotype and phenotype in the xeroderma pigmentosum-Cockayne syndrome-trichothiodystrophy spectrum Italicized letters in ovals indicate mutated genes. Rectangles are phenotypes. Because of the complexity of the relationship, it is (more...)
Testing first for founder variants present in specific parts of the world may be considered:
- XPA
- India: c.335_338delTTATinsCATAAGAAA [Tamhankar et al 2015]
- Japan: c.390-1G>C (carrier frequency of 1%) [Hirai et al 2006]
- Tunisia: p.Arg228Ter [Messaoud et al 2010]
- XPC. North Africa: c.1643_1644delTG [Soufir et al 2010, Hadj-Rabia et al 2013, Jerbi et al 2016]
- ERCC2. Iraqi Jewish: p.Arg683Gln [Falik-Zaccai et al 2012]
- POLH
- Tunisia / North Africa: Del exon 10 [Ben Rekaya et al 2014]
- Japan: c.490G>T (splice site variant); p.Ser242Ter; p.Glu306Ter and c.1661delA [Masaki et al 2008]
- Basque / Northern Spain: c.764+1G>A [Calmels et al 2016]
A multigene panel that includes DDB2, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, POLH, XPA, and XPC and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included and the sensitivity of multigene panels vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
For an introduction to multigene panels click here. More detailed information for clinicians ordering genetic tests can be found here.
More comprehensive genomic testing (when available) including exome sequencing and genome sequencing may be considered if serial single-gene testing (and/or use of a multigene panel that includes DDB2, ERCC1, ERCC2, ERCC3, ERCC4, ERCC5, POLH, XPA, and XPC) fails to confirm a diagnosis in an individual with features of XP. Such testing may provide or suggest a diagnosis not previously considered (e.g., mutation of a different gene or genes that results in a similar clinical presentation). For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Xeroderma Pigmentosum
| Gene 1 | Proportion of XP Attributed to Pathogenic Variants in Gene 2 | Proportion of Pathogenic Variants 3 Detectable by Method | |||
|---|---|---|---|---|---|
| US | Japan | Europe | Sequence analysis 4 | Gene-targeted deletion/duplication analysis 5 | |
| DDB2 | 3% | 3% | 15/15 alleles 6 | Unknown 7 | |
| ERCC1 | Rare | 4/4 alleles 8 | Unknown | ||
| ERCC2 | 28% | 5% | 16% | >99% | Unknown |
| ERCC3 | 1% | 0% | 2% | 8/8 alleles 9 | Unknown |
| ERCC4 10 | 0% | 7% | 3% | ~99% | Rare 11 |
| ERCC5 | 3% | 1% | 9% | >99% | Unknown |
| POLH | 7% | 25% | 13% | 25/40 alleles; 33/42 alleles 12 | 15/40 alleles; 9/42 alleles 12 |
| XPA | 9% | 55% 13 | 20% | 100% | Unknown |
| XPC | 43% | 3% | 31% | 7/12 alleles 14 | 5/12 alleles 14 |
- 1.
See Table A. Genes and Databases for chromosome locus and protein.
- 2.
Frequencies are taken from Bradford et al [2011] for 106 individuals with XP in the US, Moriwaki & Kraemer [2001] for 291 individuals with XP in Japan, and Fassihi et al [2016] for 89 affected individuals seen at a clinic in London, UK.
- 3.
See Molecular Genetics for information on allelic variants detected in this gene.
- 4.
Sequence analysis detects variants that are benign, likely benign, of uncertain significance, likely pathogenic, or pathogenic. Pathogenic variants may include small intragenic deletions/insertions and missense, nonsense, and splice site variants; typically, exon or whole-gene deletions/duplications are not detected. For issues to consider in interpretation of sequence analysis results, click here.
- 5.
Gene-targeted deletion/duplication analysis detects intragenic deletions or duplications. Methods used may include quantitative PCR, long-range PCR, multiplex ligation-dependent probe amplification (MLPA), and a gene-targeted microarray designed to detect single-exon deletions or duplications.
- 6.
Nichols et al [1996], Rapić-Otrin et al [2003], Fassihi et al [2016]
- 7.
No data on detection rate of gene-targeted deletion/duplication analysis are available.
- 8.
Jaspers et al [2007], Kashiyama et al [2013]
- 9.
Oh et al [2006], Fassihi et al [2016]
- 10.
Individuals with pathogenic variants in ERCC4 have been reported with phenotypes of Fanconi anemia, XP/Cockayne Syndrome complex or with combined XP/CS/FA [Bogliolo et al 2013, Kashiyama et al 2013].
- 11.
A deletion of exon 3 has been reported [Ahmad et al 2010].
- 12.
The majority of reported POLH variants are detectable by sequencing; however, single- and multiexon deletions have been reported. The frequency of deletions varies by population [Broughton et al 2002, Opletalova et al 2014]. Deletion of exon 10 accounted for 32/32 alleles in Tunisian patients with XP-V [Ben Rekaya et al 2014].
- 13.
Common in Japan, rare in the US and Europe
- 14.
Chavanne et al [2000]
Functional tests on living cells including cellular ultraviolet (UV) hypersensitivity, unscheduled DNA synthesis (UDS), and host-cell reactivation can be used to screen for abnormalities in DNA repair, but are not commonly available [Stefanini & Kraemer 2008, Kraemer & Ruenger 2012, Ruenger et al 2012].
Click here (pdf) for additional test options available on a research basis only.
Clinical Characteristics
Clinical Description
The findings in 106 individuals with XP examined at the NIH in a long-term study from 1971 to 2009 by Bradford et al [2011] are summarized below. Citations from earlier studies are provided as well.
Xeroderma pigmentosum (XP)
- Cutaneous. Approximately 60% of individuals with XP have a history of acute sunburn reaction on minimal UV exposure. The other 40% of individuals with XP tan without excessive burning [Sethi et al 2013]. In all individuals, numerous freckle-like hyperpigmented macules appear on sun-exposed skin. The median onset of the cutaneous symptoms is between ages one and two years [Kraemer et al 1987, Kraemer et al 1994]. These abnormalities are limited to sun-exposed areas.Continued sun exposure causes the skin to become dry and parchment-like with increased pigmentation; hence the name xeroderma pigmentosum ("dry pigmented skin"). Most individuals with XP develop xerosis (dry skin) and poikiloderma (the constellation of hyper- and hypopigmentation, atrophy, and telangiectasia). Premalignant actinic keratoses develop at an early age. XP is an example of accelerated photo-aging. The appearance of sun-exposed skin in children with XP is similar to that occurring in farmers and sailors after many years of extreme sun exposure.
- Ocular. Ocular abnormalities are almost as common as the cutaneous abnormalities [Kraemer et al 1987, Kraemer et al 1994]. Findings may begin in the first decade of life and are usually limited to the anterior, UV-exposed portions of the eyes: conjunctiva, cornea, and lids [Dollfus et al 2003, Brooks et al 2013]. The benign conjunctival inflammatory masses that develop can spread to obscure the cornea. Epithelioma, squamous cell carcinoma, and melanoma of UV-exposed portions of the eye are common. The ocular manifestations may be more severe in black individuals [Dollfus et al 2003, Ramkumar et al 2011, Brooks et al 2013].
- Neurologic. Progressive neurologic abnormalities that worsen slowly were reported in approximately 25% of 106 affected individuals [Rapin et al 2000, Kraemer et al 2007, DiGiovanna & Kraemer 2012, Lai et al 2013, Totonchy et al 2013, Viana et al 2013]. The onset may be early in infancy or, in some individuals, delayed until the second decade or later [Rapin et al 2000]. The neurologic abnormalities may be mild (e.g., isolated hyporeflexia) or severe, with microcephaly, progressive intellectual impairment, sensorineural hearing loss beginning with high frequencies, spasticity, ataxia, and/or seizures. During an upper respiratory infection some individuals may develop difficulty swallowing or, rarely, vocal cord paralysis [Ohto et al 2004].The predominant neuropathologic abnormality found at autopsy in individuals with neurologic symptoms is loss (or absence) of neurons, particularly in the cerebrum and cerebellum. There is evidence for a primary axonal degeneration in peripheral nerves, in some cases with secondary demyelination [Rapin et al 2000, Lai et al 2013, Viana et al 2013].Reduced nerve conduction velocity may also be present on nerve conduction studies.
- Cutaneous neoplasia. If aggressive UV avoidance is not begun early, accumulated sunlight-induced DNA damage is likely to result in skin cancer within the first decade of life. Bradford et al [2011] found that individuals with XP younger than age 20 years were at increased risk for the following cancers:
- Non-melanoma (basal cell and squamous cell) skin cancer at UV-exposed sites. The >10,000-fold increased risk was associated with a median age of onset of nine years, nearly 60 years earlier than in the US general population.
- Cutaneous melanoma. The >2000-fold increased risk was associated with the median age of onset of 22 years, more than 30 years earlier than in the US general population.
- Surprisingly, the persons with XP with the most severe sun sensitivity had a later onset of skin cancer – probably because they used greater sun protection.
Other neoplasias. Review of the world literature has revealed a substantial number of people with XP who have oral cavity neoplasms, particularly squamous cell carcinoma of the tip of the tongue, a presumed sun-exposed location [Kraemer et al 1987, Kraemer et al 1994, Butt et al 2010].
Gliomas of the brain and spinal cord, tumors of the lung, uterus, breast, pancreas, stomach, kidney, and testicles, and leukemia have been reported in a few individuals with XP [DiGiovanna et al 1998, Bradford et al 2011, Lai et al 2013, Fassihi et al 2016].
Because some of the carcinogens in cigarette smoke bind to DNA resulting in damage that is repaired by the nucleotide excision repair (NER) system, this unrepaired DNA damage may contribute to the development of lung cancer in individuals with XP. Overall, these reports suggest an approximate ten- to 20-fold increase in internal neoplasms in XP [Kraemer et al 1987, Kraemer et al 1994, Bradford et al 2011].
Causes of death. The most common causes of death were skin cancer (34%, n=10); neurologic degeneration (31%, n=9); and internal cancer (17%, n=5). The median age at death (29 years) in persons with XP with neurodegeneration was younger than that in persons with XP without neurodegeneration (37 years) (p=0.02).
Phenotype Correlations by Gene
For the overall clinical disorders (XP, Cockayne syndrome [CS], trichothiodystrophy [TTD], cerebrooculofacioskeletal [COFS] syndrome, and others [see Figure 1]), the clinical phenotypes are related within broad groups to the specific gene that is mutated (see Figure 1 and Table 2).
Table 2.
Phenotype Correlations by Gene in XP and Related Disorders
| Gene | Cutaneous Neoplasia | Phenotype |
|---|---|---|
| DDB2 | + 1 | XP with no neurologic abnormalities |
| ERCC1 | – | COFS syndrome 2 |
| ERCC2 3 | + | XP w/neurologic abnormalities ranging from none to severe |
| + | XP/CS | |
| + | XP/TTD | |
| - | TTD | |
| - | COFS syndrome | |
| ERCC3 4 | + | XP/CS |
| - | TTD | |
| + | XP w/mild neurologic abnormalities | |
| ERCC4 | + | XP w/no neurologic abnormalities or severe late-onset neurologic abnormalities 5; Fanconi anemia (FA) 6; 1 individual w/features of XP, CS, & FA, & 2 individuals w/CS 7 |
| ERCC5 | + | XP w/no neurologic abnormalities or severe neurologic abnormalities |
| + | XP/CS | |
| POLH | + | XP w/no neurologic abnormalities 8 |
| XPA | + | XP w/neurologic abnormalities ranging from mild to severe |
| XPC | + | XP w/no neurologic abnormalities 9, 10 |
COFS = cerebrooculofacioskeletal; TTD = trichothiodystrophy (without XP); XP/CS = xeroderma pigmentosum-Cockayne syndrome complex; XP/TTD = trichothiodystrophy with XP
- 1.
Adults with large numbers of skin cancers have been reported [Oh et al 2011, Fassihi et al 2016].
- 2.
Only one person with COFS syndrome has been reported to have biallelic pathogenic variants in ERCC1 [Jaspers et al 2007]. One individual with a homozygous ERCC1 pathogenic variant had severe Cockayne syndrome type II and died at age 2.5 years [Kashiyama et al 2013].
- 3.
Individuals with XP caused by mutation of ERCC2 (XP-D) have XP, XP with neurologic abnormalities, the XP/CS complex, TTD, or XP/TTD [Broughton et al 2001, Lehmann 2001, Fassihi et al 2016].
- 4.
Robbins et al [1974], Weeda et al [1997], Oh et al [2006], Fassihi et al [2016].
- 5.
Most individuals are from Japan. Several have been seen in UK [Fassihi et al 2016].
- 6.
Bogliolo et al [2013]
- 7.
Kashiyama et al [2013]
- 8.
Individuals with XP variant are clinically identical to other individuals with XP with cutaneous symptoms without neurologic abnormalities [Inui et al 2008, Fassihi et al 2016].
- 9.
"XP neurologic abnormalities" refers to progressive loss of motor, sensory, and cognitive function thought to result from neuronal loss.
- 10.
Most individuals with XP caused by mutation of XPC (XP-C) have XP without XP neurologic abnormalities [Cleaver et al 1999, Bradford et al 2011, Fassihi et al 2016].
Genotype-Phenotype Correlations
The study of genotype-phenotype correlations is ongoing. Further information is included in literature-based reviews [Cleaver et al 1999, Schubert et al 2014, Fassihi et al 2016].
Nomenclature
Xeroderma pigmentosum was first described in Vienna by Moriz Kaposi in the textbook of dermatology he published in 1870 with his father-in-law, Ferdinand Hebra. The disorder was first called xeroderma or parchment skin. See discussion in Kraemer et al [1987] and in DiGiovanna & Kraemer [2012].
Previously, an individual with XP with any neurologic abnormality was said to have the DeSanctis-Cacchione syndrome. With clarification of the spectrum of XP disease, this term is now reserved for XP with severe neurologic disease with dwarfism and immature sexual development. The complete DeSanctis-Cacchione syndrome has been recognized in very few individuals; however, many individuals with XP have one or more of its neurologic features.
"Pigmented xerodermoid" is now known to be identical to the XP variant.
Before the genes responsible for XP were identified, complementation groups were used to categorize functional defects in affected individuals. In an XP complementation analysis cells from affected individuals were fused in the laboratory to determine whether their defects were different, in which case they would be able to supply all functions necessary to restore a normal cellular phenotype. Complementation is therefore a test of function and enabled the categorization of affected individuals as having the same or different defects. Subsequently, each complementation group was found to result from a defect in a distinct gene (Table 3). Testing to assign complementation group is currently not commercially available.
Table 3.
XP-Associated Genes and Their Complementation Groups
| Gene | Complementation Group 1 |
|---|---|
| DDB2 | E |
| ERCC1 | See footnote 2 |
| ERCC2 | D |
| ERCC3 | B |
| ERCC4 | F |
| ERCC5 | G |
| POLH | Variant |
| XPA | A |
| XPC | C |
- 1.
DiGiovanna & Kraemer [2012]
- 2.
Previously called group H, a designation that was subsequently withdrawn
Prevalence
Prevalence is estimated at 1:1,000,000 in the United States and Europe [Kleijer et al 2008].
Certain populations have a higher prevalence:
- In Japan prevalence is estimated at 1:22,000 [Hirai et al 2006].
- In North Africa (Tunisia, Algeria, Morocco, Libya, and Egypt [Ben Rekaya et al 2009, Messaoud et al 2010, Soufir et al 2010] and the Middle East (Turkey, Israel, and Syria) [Kraemer & Slor 1985, Jerbi et al 2016] prevalence is increased, especially in communities in which consanguinity is common.
Differential Diagnosis
Xeroderma pigmentosum (XP), XP with neurologic abnormalities, Cockayne syndrome (CS), the XP/CS complex, trichothiodystrophy (TTD), the XP/TTD complex, cerebrooculofacioskeletal (COFS) syndrome, COFS/TTD, CS/TTD complex, and the UV-sensitive syndrome [Horibata et al 2004, Berneburg & Kraemer 2007, Kraemer et al 2007, Stefanini & Kraemer 2008, Kraemer & Ruenger 2012, Ruenger et al 2012] represent ten genetic diseases that exhibit cutaneous photosensitivity caused by defective nucleotide excision repair (NER). They are associated with defects in 13 different genes (see Figure 1).
Table 5.
Autosomal Recessive Nucleotide Excision Repair Disorders Exhibiting Cutaneous Photosensitivity
| Phenotype 1 | Gene(s) | Clinical Features |
|---|---|---|
| Cerebrooculofacioskeletal (COFS) syndrome (Pena-Shokeir syndrome, type II; OMIM PS214150) | ERCC2 2 ERCC5 2 ERCC6 3 | Progressive neurologic disorder marked by microcephaly w/intracranial calcifications & growth failure. Ocular findings of microcornea, cataracts, & optic atrophy are present along w/congenital joint contractures. Photosensitivity may occur w/a concurrent cellular phenotype of UV sensitivity. |
| Cockayne syndrome (CS) | ERCC6 ERCC8 4 | CS type I: Normal prenatal growth w/onset of growth & developmental abnormalities in first 2 years. By the time the disease has become fully manifest, height, weight, & head circumference are far below 5th %ile. Progressive impairment of vision, hearing,& central & peripheral nervous system function → severe disability. Death typically occurs in 1st or 2nd decade. As in XP, cells from individuals w/CS are hypersensitive to killing by UV; however, CS cells have normal post-UV unscheduled DNA synthesis (UDS). CS cells also have delayed recovery of RNA synthesis after UV exposure, reflecting their deficiency in transcription-coupled nucleotide excision repair (TC-NER). |
| Trichothiodystrophy (TTD; OMIM PS601675) | ERCC3 2 ERCC2 2 GTF2H5 MPLKIP 5 GTF2E2 6 | Variable phenotype incl photosensitivity, ichthyosis, brittle hair w/"tiger tail" appearance under polarizing microscopy 7, intellectual impairment, short stature, microcephaly, dysmyelination of the brain, & the characteristic facial features of protruding ears & micrognathia 20-fold ↑ risk of death before age 10 yrs, primarily from infections 8 Frequency of pregnancy complications & neonatal abnormalities is↑ in TTD but not in mothers w/XP who have different pathogenic variants in the same gene (ERCC2). 9 |
| XP/CS complex | ERCC2 2 ERCC3 2 ERCC4 2 ERCC5 10, 11 | Facial freckling & early skin cancers typical of XP & some features of CS (e.g., intellectual disability, spasticity, short stature, hypogonadism) but not skeletal dysplasia. In contrast to XP, in which neuronal degeneration predominates, dysmyelination typical of CS is observed in XP/CS. |
| COFS/TTD | ERCC2 12 | Combined features of COFS & TTD |
| CS/TTD complex | ERCC2 12 | Combined features of CS & TTD |
| UV-sensitive syndrome | ERCC6 13 ERCC8 13 UVSSA 13 | Mild photosensitivity w/out pigmentary abnormalities or apparent defects in the central nervous system. Cells from affected individuals have the same transcription defects as those in individuals w/CS. |
| XP/TTD complex | ERCC2 14 | Phenotypic features of TTD w/the clinical & cellular phenotype of XP. Unlike most people w/TTD, those w/XP/TTD may experience ↑ frequency of skin cancers. |
AR = autosomal recessive; MOI = mode of inheritance
- 1.
See hyperlinked GeneReview, OMIM phenotype entry, or cited reference for more information.
- 2.
Allelic with XP
- 3.
Meira et al [2000], Graham et al [2001]
- 4.
Two individuals with Cockayne syndrome and one individual with phenotypic features of Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia had biallelic pathogenic variants in either ERCC1 or ERCC4 [Kashiyama et al 2013].
- 5.
Broughton et al [2001], Itin et al [2001], Bootsma et al [2002], Giglia-Mari et al [2004], Liang et al [2005], Kraemer et al [2007], Heller et al [2015]
- 6.
Kuschal et al [2016]
- 7.
Liang et al [2005]
- 8.
Faghri et al [2008]
- 9.
Moslehi et al [2010], Tamura et al [2011], Tamura et al [2012]
- 10.
See Table 2 and Figure 1.
- 11.
Kashiyama et al [2013]
- 12.
See DiGiovanna & Kraemer [2012]
- 13.
Itoh et al [1994], Horibata et al [2004], Nardo et al [2009], Nakazawa et al [2012], Schwertman et al [2012], Zhang et al [2012], Wilson et al [2016]
- 14.
Broughton et al [2001], Boyle et al [2008], DiGiovanna & Kraemer [2012]
Other
In addition to diseases sharing deficient nucleotide excision repair, other diseases exhibiting cutaneous photosensitivity may be considered, especially in cases with a paucity of other clinical findings. These other diseases include the following:
- Rothmund-Thomson syndrome and the allelic disorder Baller-Gerold syndrome
- Hartnup disorder (OMIM 234500), a disorder of amino acid absorption resulting from biallelic pathogenic variants in SLC6A19, a non-polar amino acid transporter. Affected individuals may have reduced levels of niacin with resulting pellagra-like symptoms of photosensitivity with dermatitis, diarrhea, and dementia. However, individuals with Hartnup disorder are not reported to have increased frequency of skin cancer, as is seen in those with XP.
The cutaneous findings of Carney complex may be confused with those of XP; however, Carney complex is characterized by lentigines without evidence of the usually associated signs of skin damage such as atrophy and telangiectasia (i.e., poikiloderma) and cutaneous findings are not limited to sun-exposed sites [Correa et al 2015].
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with xeroderma pigmentosum (XP), the following evaluations are recommended (reviewed in Tamura et al [2014]).
Skin
- Perform baseline examination of the skin (including all sun-exposed as well as sun-shielded areas) for evidence of sunlight-induced damage including pigmentary changes, precancerous lesions, and skin cancers.
- For examination of the scalp, use a hair dryer (on a cool setting) to blow the hair aside.
- Examination of the lip and adjacent tip of the tongue for signs of sun damage, including actinic cheilitis (a type of actinic keratosis or leukoplakia occurring on the lips) and prominent telangiectasia, which may precede the development of cancer in these areas [Butt et al 2010].
- Baseline clinical color photographs of the entire skin surface with close-ups (including a ruler) of individual lesions to facilitate follow-up and detection of early skin cancers.
Eyes
- Examine the lids and anterior UV-exposed portions of the globe for evidence of sun-induced damage including ectropion, entropion, inflammatory masses (pterygia, pinguecula), clouding of the cornea, and cancer of the lids, conjunctiva, or cornea. Eversion of the lids may be necessary to detect cancers of the mucosal surface.
- Use the Schirmer test to detect dry eyes. This test involves measurement of the extent of absorption of tears into filter paper placed under eyelids for a few minutes [Brooks et al 2013].
Neurologic
- Deep tendon reflex testing
- Measurement of the occipital frontal circumference (OFC), to determine if microcephaly is present
- MRI of the brain and nerve conduction velocities, if other neurologic problems are detected
Auditory. Baseline audiometry evaluation to screen for the sensorineural hearing loss, which may be present as a component of the XP-associated neurologic abnormalities [Totonchy et al 2013]
Genetic. Consultation with a clinical geneticist and/or genetic counselor
Treatment of Manifestations
The treatment of manifestations is reviewed in Tamura et al [2010b] and Tamura et al [2014].
Skin. Premalignant lesions (e.g., small actinic keratosis) may be treated by freezing with liquid nitrogen.
Larger areas of sun-damaged skin can be treated with field treatments such as topical 5-fluorouracil or imiquimod preparations. Rarely, therapeutic dermatome shaving or dermabrasion has been used to remove the more damaged superficial epidermal layers. This procedure permits repopulation by relatively UV-shielded cells from the follicles and glands.
Cutaneous neoplasms are treated in the same manner as in individuals who do not have XP. This involves electrodesiccation/curettage or surgical excision. Skin cancers which are recurrent or in locations at high risk for recurrence are best treated with Mohs micrographic surgery. Because multiple surgical procedures are often necessary, removal of undamaged skin should be minimized. Severe cases have been treated by excision of large portions of the facial surface and grafting with sun-protected skin.
While individuals with XP are not abnormally sensitive to therapeutic x-rays, and individuals with XP have responded normally to full-dose therapeutic x-radiation for treatment of inoperable neoplasms [DiGiovanna et al 1998], cultured cells from a few individuals with XP were found to be hypersensitive to x-radiation [Arlett et al 2006]. When x-radiation therapy is indicated, an initial small dose is advisable to test for clinical hypersensitivity.
Oral isotretinoin or acitretin can be effective in preventing new neoplasms in individuals with multiple skin cancers [Kraemer et al 1988]. Because of its toxicity (hepatic, hyperlipidemic, and teratogenic effects; calcification of ligaments and tendons; premature closure of the epiphyses), oral isotretinoin or acitretin should be reserved for individuals with XP who are actively developing large numbers of new tumors. Some individuals may respond to lower doses of isotretinoin or acitretin with less toxicity.
A few case reports have described regression of skin cancers with use of imiquimod cream in individuals with XP [Giannotti et al 2003, Nagore et al 2003, Roseeuw 2003]; however, no controlled studies have been reported.
Eyes. Methylcellulose eye drops or soft contact lenses have been used to keep the cornea moist and to protect against mechanical trauma in individuals with deformed eyelids.
Corneal transplantation has restored vision in individuals with severe keratitis with corneal opacity. However, the immunosuppression necessary to prevent rejection of the transplant may increase the risk for skin cancer.
Neoplasms of the lids, conjunctiva, and cornea are usually treated surgically.
Hearing. Hearing aids can be of great help for individuals who have sensorineural hearing loss with learning difficulties in school (see Totonchy et al [2013] and Deafness and Hereditary Hearing Loss Overview).
Prevention of Primary Manifestations
Treatment of XP depends on early diagnosis and immediate, aggressive avoidance of sun and other UV exposure. This involves avoiding or minimizing outdoor exposure at times when UV radiation is present (when the sun is out or during daytime through clouds).
Clinical suspicion of XP should prompt immediate sun-protective measures until the diagnosis is confirmed or an alternative explanation is determined.
Individuals should be educated to protect all body surfaces from UV radiation by wearing protective clothing including hats, long sleeves, long pants and gloves, broad-spectrum, high sun-protective factor (SPF) sunscreens, UV-absorbing glasses, and long hair styles. The eyes should be protected by wearing UV-absorbing glasses with side shields. Some individuals have custom-made hats with UV-absorbing face shields to permit visibility outdoors while protecting the face from UV.
Because the cells of individuals with XP are hypersensitive to UVA and UVB (found in sunlight) and UVC (found in some artificial light sources), it is useful to measure UV light in an individual's home, school, or work environment with a light meter so that high levels of environmental UV (e.g., halogen lamps) can be identified and eliminated if possible. While no standards exist for perfectly safe UV exposure in individuals with XP, the use of UV meters can alert individuals to unexpected sources of high levels of environmental UV.
Prevention of Secondary Complications
Vitamin D is produced in the skin by a reaction involving exposure to UV radiation. Active adults with XP and skin cancers received sufficient vitamin D in their diet in the past to result in normal serum concentrations of the active form (1,25 dihydroxy vitamin D) [Sollitto et al 1997]. However, children protected from sunlight very early in life have had low serum concentration of 25 hydroxy vitamin D; one child became susceptible to bone fractures [Ali et al 2009; Author, personal observation]. Dietary supplementation with oral vitamin D is recommended for persons with low serum concentration of serum vitamin D [Reichrath 2007; Author, personal communication].
Surveillance
See Tamura et al [2010b] and Tamura et al [2014].
Skin. A physician should examine the skin of an affected individual at frequent intervals (every ~3-12 months, depending on the severity of skin disease).
Affected individuals or their parents should be educated to look for abnormal pigmented lesions or the appearance of basal cell or squamous cell carcinoma. Individuals should be examined frequently by a family member who has been instructed in recognition of cutaneous neoplasms.
Eyes should be examined regularly for signs of UV exposure and damage.
Neurologic. Routine neurologic examination is indicated because of progressive neurologic abnormalities that are present in a minority of individuals with XP and may not be detected in young children.
Hearing. Periodic audiograms. Serial audiograms at regular intervals may also be useful for assessing the presence or absence of progressive neurologic degeneration, especially in those with a history of acute burning on minimal sun exposure [Totonchy et al 2013].
Agents/Circumstances to Avoid
UV exposure from sunlight and artificial sources of UV radiation should be avoided (see Prevention of Primary Manifestations).
Artificial sources of UV. Certain light sources (e.g., mercury arc, halogen, and other lamps) can be unrecognized sources of UV. Although such light sources are often shielded, in open areas such as gymnasiums they can be a source of UV if the shield has been breached. UV meters are readily available to enable monitoring of areas to identify unexpected UV sources.
Cigarette smoke. Because cells from individuals with XP are also hypersensitive to environmental mutagens, such as benzo[a]pyrene found in cigarette smoke, prudence dictates that individuals with XP should be protected against these agents. One individual with XP who smoked cigarettes for more than ten years died of bronchogenic carcinoma of the lungs at age 35 years [Kraemer et al 1994]. The authors recently cared for another individual with XP who smoked and developed lung cancer in the fifth decade of life.
Evaluation of Relatives at Risk
It is appropriate to evaluate the apparently asymptomatic older and younger sibs of a proband in order to identify as early as possible those who would benefit from prompt initiation of treatment and preventive measures.
- If the family-specific pathogenic variants have been identified, molecular genetic testing for at-risk sibs is possible.
- Clinical evaluation to identify affected sibs of a proband may be difficult, especially in an infant or young child. In this case, sun protection may be recommended for sibs until a definitive laboratory diagnosis is obtained.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
The systemic retinoids isotretinoin and acitretin are used as skin cancer chemopreventive agents in individuals who are actively developing large numbers of skin cancers, and thus may be used by some women with XP [Kraemer et al 1988]. Systemic retinoids are known to be teratogenic to a developing fetus and pose a high risk for birth defects. Therefore, women who are using systemic retinoids should be appropriately counseled about pregnancy risks and the need for effective contraception; regular monitoring with pregnancy tests is indicated. Systemic retinoids should be administered only by physicians who are knowledgeable regarding their risks and benefits.
To access isotretinoin in the US, women and their prescribing providers must be enrolled in the iPLEDGE program to minimize the potential for fetal exposure. Pregnancy avoidance is initiated before therapy, continues during therapy, and extends post-treatment until the drug is cleared from the body. While both drugs may be effective in preventing skin cancers, acitretin may take longer to be eliminated from the body, requiring an extended period (3 years) of post-therapy pregnancy avoidance to minimize teratogenic risk.
Therapies Under Investigation
The bacterial DNA repair enzyme T4 endonuclease V in a topical liposome-containing preparation has been reported to reduce the frequency of new actinic keratoses and basal cell carcinomas in individuals with XP in one research study [Yarosh et al 2001]. As of 2016, this treatment is not approved by the US Food and Drug Administration.
Oral vismodegib (Erivedge®), an inhibitor of the hedgehog pathway, has been approved by the FDA for treatment of metastatic basal cell carcinoma or locally advanced basal cell carcinoma that has recurred following surgery. This drug has also been approved for use in individuals with basal cell carcinoma who are not candidates for surgery or for radiation therapy (see FDA package insert). This treatment may be appropriate for some individuals with XP, however, no studies on the efficacy of this drug in those with XP have been published. Oral vismodegib is also a teratogen, leading to embryo-fetal death, midline defects, missing digits, and other birth defects in an exposed embryo or fetus; effective contraception during and after vismodegib treatment is advised in both women and men.
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for access to information on clinical studies for a wide range of diseases and conditions.
Other
Use of cotton swabs to obtain specimens from conjunctival lesions for cytologic examination for malignant cells is being evaluated [Brooks et al 2013].