Ebola

Ebola, also known as Ebola virus disease (EVD) or Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever of humans and other primates caused by ebolaviruses. Signs and symptoms typically start between two days and three weeks after contracting the virus with a fever, sore throat, muscular pain, and headaches. Vomiting, diarrhoea and rash usually follow, along with decreased function of the liver and kidneys. At this time, some people begin to bleed both internally and externally. The disease has a high risk of death, killing 25% to 90% of those infected, with an average of about 50%. This is often due to low blood pressure from fluid loss, and typically follows six to 16 days after symptoms appear.
The virus spreads through direct contact with body fluids, such as blood from infected humans or other animals. Spread may also occur from contact with items recently contaminated with bodily fluids. Spread of the disease through the air between primates, including humans, has not been documented in either laboratory or natural conditions. Semen or breast milk of a person after recovery from EVD may carry the virus for several weeks to months. Fruit bats are believed to be the normal carrier in nature, able to spread the virus without being affected by it. Other diseases such as malaria, cholera, typhoid fever, meningitis and other viral haemorrhagic fevers may resemble EVD. Blood samples are tested for viral RNA, viral antibodies or for the virus itself to confirm the diagnosis.
Control of outbreaks requires coordinated medical services and community engagement. This includes rapid detection, contact tracing of those who have been exposed, quick access to laboratory services, care for those infected, and proper disposal of the dead through cremation or burial. Samples of body fluids and tissues from people with the disease should be handled with special caution. Prevention includes limiting the spread of disease from infected animals to humans by handling potentially infected bushmeat only while wearing protective clothing, and by thoroughly cooking bushmeat before eating it. It also includes wearing proper protective clothing and washing hands when around a person with the disease. An Ebola vaccine was approved in the United States in December 2019. While there is no approved treatment for Ebola as of 2019[update], two treatments (REGN-EB3 and mAb114) are associated with improved outcomes. Supportive efforts also improve outcomes. This includes either oral rehydration therapy (drinking slightly sweetened and salty water) or giving intravenous fluids as well as treating symptoms. Atoltivimab/maftivimab/odesivimab (Inmazeb) was approved for medical use in the United States in October 2020, for the treatment of infection caused by Zaire ebolavirus.
The disease was first identified in 1976, in two simultaneous outbreaks: one in Nzara (a town in South Sudan) and the other in Yambuku (Democratic Republic of the Congo), a village relatively near the Ebola River from which the disease takes its name. EVD outbreaks occur intermittently in tropical regions of sub-Saharan Africa. From 1976 to 2012, the World Health Organization reports 24 outbreaks involving 2,387 cases with 1,590 deaths. The largest outbreak to date was the epidemic in West Africa, which occurred from December 2013 to January 2016, with 28,646 cases and 11,323 deaths. It was declared no longer an emergency on 29 March 2016. Other outbreaks in Africa began in the Democratic Republic of the Congo in May 2017, and 2018. In July 2019, the World Health Organization declared the Congo Ebola outbreak a world health emergency.
Signs and symptoms
Onset
The length of time between exposure to the virus and the development of symptoms (incubation period) is between two and 21 days, and usually between four and ten days. However, recent estimates based on mathematical models predict that around 5% of cases may take longer than 21 days to develop.
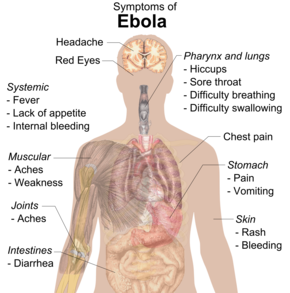
Symptoms usually begin with a sudden influenza-like stage characterised by feeling tired, fever, weakness, decreased appetite, muscular pain, joint pain, headache, and sore throat. The fever is usually higher than 38.3 °C (101 °F). This is often followed by nausea, vomiting, diarrhoea, abdominal pain, and sometimes hiccups. The combination of severe vomiting and diarrhoea often leads to severe dehydration. Next, shortness of breath and chest pain may occur, along with swelling, headaches, and confusion. In about half of the cases, the skin may develop a maculopapular rash, a flat red area covered with small bumps, five to seven days after symptoms begin.
Bleeding
In some cases, internal and external bleeding may occur. This typically begins five to seven days after the first symptoms. All infected people show some decreased blood clotting. Bleeding from mucous membranes or from sites of needle punctures has been reported in 40–50% of cases. This may cause vomiting blood, coughing up of blood, or blood in stool. Bleeding into the skin may create petechiae, purpura, ecchymoses or haematomas (especially around needle injection sites). Bleeding into the whites of the eyes may also occur. Heavy bleeding is uncommon; if it occurs, it is usually in the gastrointestinal tract. The incidence of bleeding into the gastrointestinal tract was reported to be ~58% in the 2001 outbreak in Gabon, but in the 2014–15 outbreak in the US it was ~18%, possibly due to improved prevention of disseminated intravascular coagulation.
Recovery and death
Recovery may begin between seven and 14 days after first symptoms. Death, if it occurs, follows typically six to sixteen days from first symptoms and is often due to low blood pressure from fluid loss. In general, bleeding often indicates a worse outcome, and blood loss may result in death. People are often in a coma near the end of life.
Those who survive often have ongoing muscular and joint pain, liver inflammation, and decreased hearing, and may have continued tiredness, continued weakness, decreased appetite, and difficulty returning to pre-illness weight. Problems with vision may develop. It is recommended that survivors of EVD wear condoms for at least twelve months after initial infection or until the semen of a male survivor tests negative for Ebola virus on two separate occasions.
Survivors develop antibodies against Ebola that last at least 10 years, but it is unclear whether they are immune to additional infections.
Cause
EVD in humans is caused by four of five viruses of the genus Ebolavirus. The four are Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV) and one simply called Ebola virus (EBOV, formerly Zaire Ebola virus). EBOV, species Zaire ebolavirus, is the most dangerous of the known EVD-causing viruses, and is responsible for the largest number of outbreaks. The fifth virus, Reston virus (RESTV), is not thought to cause disease in humans, but has caused disease in other primates. All five viruses are closely related to marburgviruses.
Virology
 e6/Ebola_virus_virion.jpg/220px-Ebola_virus_virion.jpg" decoding="async" width="220" height="101" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Ebola_virus_virion.jpg/330px-Ebola_virus_virion.jpg 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Ebola_virus_virion.jpg/440px-Ebola_virus_virion.jpg 2x" data-file-width="3679" data-file-height="1692">
e6/Ebola_virus_virion.jpg/220px-Ebola_virus_virion.jpg" decoding="async" width="220" height="101" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Ebola_virus_virion.jpg/330px-Ebola_virus_virion.jpg 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/e/e6/Ebola_virus_virion.jpg/440px-Ebola_virus_virion.jpg 2x" data-file-width="3679" data-file-height="1692"> Ebolaviruses contain single-stranded, non-infectious RNA genomes. Ebolavirus genomes contain seven genes including 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR. The genomes of the five different ebolaviruses (BDBV, EBOV, RESTV, SUDV and TAFV) differ in sequence and the number and location of gene overlaps. As with all filoviruses, ebolavirus virions are filamentous particles that may appear in the shape of a shepherd's crook, of a "U" or of a "6," and they may be coiled, toroid or branched. In general, ebolavirions are 80 nanometers (nm) in width and may be as long as 14,000 nm.
Their life cycle is thought to begin with a virion attaching to specific cell-surface receptors such as C-type lectins, DC-SIGN, or integrins, which is followed by fusion of the viral envelope with cellular membranes. The virions taken up by the cell then travel to acidic endosomes and lysosomes where the viral envelope glycoprotein GP is cleaved. This processing appears to allow the virus to bind to cellular proteins enabling it to fuse with internal cellular membranes and release the viral nucleocapsid. The Ebolavirus structural glycoprotein (known as GP1,2) is responsible for the virus' ability to bind to and infect targeted cells. The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-strand mRNAs, which are then translated into structural and nonstructural proteins. The most abundant protein produced is the nucleoprotein, whose concentration in the host cell determines when L switches from gene transcription to genome replication. Replication of the viral genome results in full-length, positive-strand antigenomes that are, in turn, transcribed into genome copies of negative-strand virus progeny. Newly synthesised structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane. Virions bud off from the cell, gaining their envelopes from the cellular membrane from which they bud. The mature progeny particles then infect other cells to repeat the cycle. The genetics of the Ebola virus are difficult to study because of EBOV's virulent characteristics.
Transmission

It is believed that between people, Ebola disease spreads only by direct contact with the blood or other body fluids of a person who has developed symptoms of the disease. Body fluids that may contain Ebola viruses include saliva, mucus, vomit, feces, sweat, tears, breast milk, urine and semen. The WHO states that only people who are very sick are able to spread Ebola disease in saliva, and the virus has not been reported to be transmitted through sweat. Most people spread the virus through blood, feces and vomit. Entry points for the virus include the nose, mouth, eyes, open wounds, cuts and abrasions. Ebola may be spread through large droplets; however, this is believed to occur only when a person is very sick. This contamination can happen if a person is splashed with droplets. Contact with surfaces or objects contaminated by the virus, particularly needles and syringes, may also transmit the infection. The virus is able to survive on objects for a few hours in a dried state, and can survive for a few days within body fluids outside of a person.
The Ebola virus may be able to persist for more than three months in the semen after recovery, which could lead to infections via sexual intercourse. Virus persistence in semen for over a year has been recorded in a national screening programme. Ebola may also occur in the breast milk of women after recovery, and it is not known when it is safe to breastfeed again. The virus was also found in the eye of one patient in 2014, two months after it was cleared from his blood. Otherwise, people who have recovered are not infectious.
The potential for widespread infections in countries with medical systems capable of observing correct medical isolation procedures is considered low. Usually when someone has symptoms of the disease, they are unable to travel without assistance.
Dead bodies remain infectious; thus, people handling human remains in practices such as traditional burial rituals or more modern processes such as embalming are at risk. 69% of the cases of Ebola infections in Guinea during the 2014 outbreak are believed to have been contracted via unprotected (or unsuitably protected) contact with infected corpses during certain Guinean burial rituals.
Health-care workers treating people with Ebola are at greatest risk of infection. The risk increases when they do not have appropriate protective clothing such as masks, gowns, gloves and eye protection; do not wear it properly; or handle contaminated clothing incorrectly. This risk is particularly common in parts of Africa where the disease mostly occurs and health systems function poorly. There has been transmission in hospitals in some African countries that reuse hypodermic needles. Some health-care centres caring for people with the disease do not have running water. In the United States the spread to two medical workers treating infected patients prompted criticism of inadequate training and procedures.
Human-to-human transmission of EBOV through the air has not been reported to occur during EVD outbreaks, and airborne transmission has only been demonstrated in very strict laboratory conditions, and then only from pigs to primates, but not from primates to primates. Spread of EBOV by water, or food other than bushmeat, has not been observed. No spread by mosquitos or other insects has been reported. Other possible methods of transmission are being studied.
Airborne transmission among humans is theoretically possible due to the presence of Ebola virus particles in saliva, which can be discharged into the air with a cough or sneeze, but observational data from previous epidemics suggests the actual risk of airborne transmission is low. A number of studies examining airborne transmission broadly concluded that transmission from pigs to primates could happen without direct contact because, unlike humans and primates, pigs with EVD get very high ebolavirus concentrations in their lungs, and not their bloodstream. Therefore, pigs with EVD can spread the disease through droplets in the air or on the ground when they sneeze or cough. By contrast, humans and other primates accumulate the virus throughout their body and specifically in their blood, but not very much in their lungs. It is believed that this is the reason researchers have observed pig to primate transmission without physical contact, but no evidence has been found of primates being infected without actual contact, even in experiments where infected and uninfected primates shared the same air.
Initial case
 e4/Bushmeat_-_Buschfleisch_Ghana.JPG/220px-Bushmeat_-_Buschfleisch_Ghana.JPG" decoding="async" width="220" height="165" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/e/e4/Bushmeat_-_Buschfleisch_Ghana.JPG/330px-Bushmeat_-_Buschfleisch_Ghana.JPG 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/e/e4/Bushmeat_-_Buschfleisch_Ghana.JPG/440px-Bushmeat_-_Buschfleisch_Ghana.JPG 2x" data-file-width="3781" data-file-height="2835">
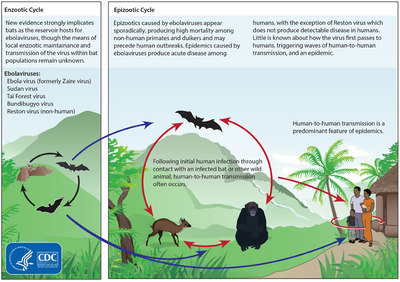
e4/Bushmeat_-_Buschfleisch_Ghana.JPG/220px-Bushmeat_-_Buschfleisch_Ghana.JPG" decoding="async" width="220" height="165" class="thumbimage" srcset="//upload.wikimedia.org/wikipedia/commons/thumb/e/e4/Bushmeat_-_Buschfleisch_Ghana.JPG/330px-Bushmeat_-_Buschfleisch_Ghana.JPG 1.5x, //upload.wikimedia.org/wikipedia/commons/thumb/e/e4/Bushmeat_-_Buschfleisch_Ghana.JPG/440px-Bushmeat_-_Buschfleisch_Ghana.JPG 2x" data-file-width="3781" data-file-height="2835"> Although it is not entirely clear how Ebola initially spreads from animals to humans, the spread is believed to involve direct contact with an infected wild animal or fruit bat. Besides bats, other wild animals sometimes infected with EBOV include several species of monkeys such as baboons, great apes (chimpanzees and gorillas), and duikers (a species of antelope).
Animals may become infected when they eat fruit partially eaten by bats carrying the virus. Fruit production, animal behavior and other factors may trigger outbreaks among animal populations.
Evidence indicates that both domestic dogs and pigs can also be infected with EBOV. Dogs do not appear to develop symptoms when they carry the virus, and pigs appear to be able to transmit the virus to at least some primates. Although some dogs in an area in which a human outbreak occurred had antibodies to EBOV, it is unclear whether they played a role in spreading the disease to people.
Reservoir
The natural reservoir for Ebola has yet to be confirmed; however, bats are considered to be the most likely candidate. Three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti and Myonycteris torquata) were found to possibly carry the virus without getting sick. As of 2013[update], whether other animals are involved in its spread is not known. Plants, arthropods, rodents, and birds have also been considered possible viral reservoirs.
Bats were known to roost in the cotton factory in which the first cases of the 1976 and 1979 outbreaks were observed, and they have also been implicated in Marburg virus infections in 1975 and 1980. Of 24 plant and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected. The bats displayed no clinical signs of disease, which is considered evidence that these bats are a reservoir species of EBOV. In a 2002–2003 survey of 1,030 animals including 679 bats from Gabon and the Republic of the Congo, immunoglobulin G (IgG) immune defense molecules indicative of Ebola infection were found in three bat species; at various periods of study, between 2.2 and 22.6% of bats were found to contain both RNA sequences and IgG molecules indicating Ebola infection. Antibodies against Zaire and Reston viruses have been found in fruit bats in Bangladesh, suggesting that these bats are also potential hosts of the virus and that the filoviruses are present in Asia.
Between 1976 and 1998, in 30,000 mammals, birds, reptiles, amphibians and arthropods sampled from regions of EBOV outbreaks, no Ebola virus was detected apart from some genetic traces found in six rodents (belonging to the species Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic. However, further research efforts have not confirmed rodents as a reservoir. Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high rates of death in these species resulting from EBOV infection make it unlikely that these species represent a natural reservoir for the virus.
Deforestation has been mentioned as a possible contributor to recent outbreaks, including the West African Ebola virus epidemic. Index cases of EVD have often been close to recently deforested lands.
Pathophysiology
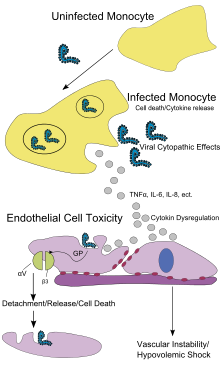
Like other filoviruses, EBOV replicates very efficiently in many cells, producing large amounts of virus in monocytes, macrophages, dendritic cells and other cells including liver cells, fibroblasts, and adrenal gland cells. Viral replication triggers high levels of inflammatory chemical signals and leads to a septic state.
EBOV is thought to infect humans through contact with mucous membranes or skin breaks. After infection, endothelial cells (cells lining the inside of blood vessels), liver cells, and several types of immune cells such as macrophages, monocytes, and dendritic cells are the main targets of attack. Following infection, immune cells carry the virus to nearby lymph nodes where further reproduction of the virus takes place. From there the virus can enter the bloodstream and lymphatic system and spread throughout the body. Macrophages are the first cells infected with the virus, and this infection results in programmed cell death. Other types of white blood cells, such as lymphocytes, also undergo programmed cell death leading to an abnormally low concentration of lymphocytes in the blood. This contributes to the weakened immune response seen in those infected with EBOV.
Endothelial cells may be infected within three days after exposure to the virus. The breakdown of endothelial cells leading to blood vessel injury can be attributed to EBOV glycoproteins. This damage occurs due to the synthesis of Ebola virus glycoprotein (GP), which reduces the availability of specific integrins responsible for cell adhesion to the intercellular structure and causes liver damage, leading to improper clotting. The widespread bleeding that occurs in affected people causes swelling and shock due to loss of blood volume. The dysfunctional bleeding and clotting commonly seen in EVD has been attributed to increased activation of the extrinsic pathway of the coagulation cascade due to excessive tissue factor production by macrophages and monocytes.
After infection, a secreted glycoprotein, small soluble glycoprotein (sGP or GP) is synthesised. EBOV replication overwhelms protein synthesis of infected cells and the host immune defences. The GP forms a trimeric complex, which tethers the virus to the endothelial cells. The sGP forms a dimeric protein that interferes with the signalling of neutrophils, another type of white blood cell. This enables the virus to evade the immune system by inhibiting early steps of neutrophil activation.
Immune system evasion
Filoviral infection also interferes with proper functioning of the innate immune system. EBOV proteins blunt the human immune system's response to viral infections by interfering with the cells' ability to produce and respond to interferon proteins such as interferon-alpha, interferon-beta, and interferon gamma.
The VP24 and VP35 structural proteins of EBOV play a key role in this interference. When a cell is infected with EBOV, receptors located in the cell's cytosol (such as RIG-I and MDA5) or outside of the cytosol (such as Toll-like receptor 3 (TLR3), TLR7, TLR8 and TLR9) recognise infectious molecules associated with the virus. On TLR activation, proteins including interferon regulatory factor 3 and interferon regulatory factor 7 trigger a signalling cascade that leads to the expression of type 1 interferons. The type 1 interferons are then released and bind to the IFNAR1 and IFNAR2 receptors expressed on the surface of a neighbouring cell. Once interferon has bound to its receptors on the neighbouring cell, the signalling proteins STAT1 and STAT2 are activated and move to the cell's nucleus. This triggers the expression of interferon-stimulated genes, which code for proteins with antiviral properties. EBOV's V24 protein blocks the production of these antiviral proteins by preventing the STAT1 signalling protein in the neighbouring cell from entering the nucleus. The VP35 protein directly inhibits the production of interferon-beta. By inhibiting these immune responses, EBOV may quickly spread throughout the body.
Diagnosis
When EVD is suspected, travel, work history, and exposure to wildlife are important factors with respect to further diagnostic efforts.
Laboratory testing
Possible non-specific laboratory indicators of EVD include a low platelet count; an initially decreased white blood cell count followed by an increased white blood cell count; elevated levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST); and abnormalities in blood clotting often consistent with disseminated intravascular coagulation (DIC) such as a prolonged prothrombin time, partial thromboplastin time, and bleeding time. Filovirions such as EBOV may be identified by their unique filamentous shapes in cell cultures examined with electron microscopy.
The specific diagnosis of EVD is confirmed by isolating the virus, detecting its RNA or proteins, or detecting antibodies against the virus in a person's blood. Isolating the virus by cell culture, detecting the viral RNA by polymerase chain reaction (PCR) and detecting proteins by enzyme-linked immunosorbent assay (ELISA) are methods best used in the early stages of the disease and also for detecting the virus in human remains. Detecting antibodies against the virus is most reliable in the later stages of the disease and in those who recover. IgM antibodies are detectable two days after symptom onset and IgG antibodies can be detected six to 18 days after symptom onset. During an outbreak, isolation of the virus with cell culture methods is often not feasible. In field or mobile hospitals, the most common and sensitive diagnostic methods are real-time PCR and ELISA. In 2014, with new mobile testing facilities deployed in parts of Liberia, test results were obtained 3–5 hours after sample submission. In 2015, a rapid antigen test which gives results in 15 minutes was approved for use by WHO. It is able to confirm Ebola in 92% of those affected and rule it out in 85% of those not affected.
Differential diagnosis
Early symptoms of EVD may be similar to those of other diseases common in Africa, including malaria and dengue fever. The symptoms are also similar to those of other viral haemorrhagic fevers such as Marburg virus disease, Crimean–Congo haemorrhagic fever, and Lassa fever.
The complete differential diagnosis is extensive and requires consideration of many other infectious diseases such as typhoid fever, shigellosis, rickettsial diseases, cholera, sepsis, borreliosis, EHEC enteritis, leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, measles, and viral hepatitis among others.
Non-infectious diseases that may result in symptoms similar to those of EVD include acute promyelocytic leukaemia, haemolytic uraemic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary haemorrhagic telangiectasia, Kawasaki disease, and warfarin poisoning.
Prevention
Vaccines
An Ebola vaccine, rVSV-ZEBOV, was approved in the United States in December 2019. It appears to be fully effective ten days after being given. It was studied in Guinea between 2014, and 2016. More than 100,000 people have been vaccinated against Ebola as of 2019[update].
Infection control

Caregivers

People who care for those infected with Ebola should wear protective clothing including masks, gloves, gowns and goggles. The U.S. Centers for Disease Control (CDC) recommend that the protective gear leaves no skin exposed. These measures are also recommended for those who may handle objects contaminated by an infected person's body fluids. In 2014, the CDC began recommending that medical personnel receive training on the proper suit-up and removal of personal protective equipment (PPE); in addition, a designated person, appropriately trained in biosafety, should be watching each step of these procedures to ensure they are done correctly. In Sierra Leone, the typical training period for the use of such safety equipment lasts approximately 12 days.
Patients and household members
The infected person should be in barrier-isolation from other people. All equipment, medical waste, patient waste and surfaces that may have come into contact with body fluids need to be disinfected. During the 2014 outbreak, kits were put together to help families treat Ebola disease in their homes, which included protective clothing as well as chlorine powder and other cleaning supplies. Education of caregivers in these techniques, and providing such barrier-separation supplies has been a priority of Doctors Without Borders.
Disinfection
Ebolaviruses can be eliminated with heat (heating for 30 to 60 minutes at 60 °C or boiling for five minutes). To disinfect surfaces, some lipid solvents such as some alcohol-based products, detergents, sodium hypochlorite (bleach) or calcium hypochlorite (bleaching powder), and other suitable disinfectants may be used at appropriate concentrations.
General population
Education of the general public about the risk factors for Ebola infection and of the protective measures individuals may take to prevent infection is recommended by the World Health Organization. These measures include avoiding direct contact with infected people and regular hand washing using soap and water.
Bushmeat
Bushmeat, an important source of protein in the diet of some Africans, should be handled and prepared with appropriate protective clothing and thoroughly cooked before consumption. Some research suggests that an outbreak of Ebola disease in the wild animals used for consumption may result in a corresponding human outbreak. Since 2003, such animal outbreaks have been monitored to predict and prevent Ebola outbreaks in humans.
Corpses, burial
If a person with Ebola disease dies, direct contact with the body should be avoided. Certain burial rituals, which may have included making various direct contacts with a dead body, require reformulation so that they consistently maintain a proper protective barrier between the dead body and the living. Social anthropologists may help find alternatives to traditional rules for burials.
Transport, travel, contact
Transportation crews are instructed to follow a certain isolation procedure, should anyone exhibit symptoms resembling EVD. As of August 2014[update], the WHO does not consider travel bans to be useful in decreasing spread of the disease. In October 2014, the CDC defined four risk levels used to determine the level of 21-day monitoring for symptoms and restrictions on public activities. In the United States, the CDC recommends that restrictions on public activity, including travel restrictions, are not required for the following defined risk levels:
- having been in a country with widespread Ebola disease transmission and having no known exposure (low risk); or having been in that country more than 21 days ago (no risk)
- encounter with a person showing symptoms; but not within three feet of the person with Ebola without wearing PPE; and no direct contact with body fluids
- having had brief skin contact with a person showing symptoms of Ebola disease when the person was believed to be not very contagious (low risk)
- in countries without widespread Ebola disease transmission: direct contact with a person showing symptoms of the disease while wearing PPE (low risk)
- contact with a person with Ebola disease before the person was showing symptoms (no risk).
The CDC recommends monitoring for the symptoms of Ebola disease for those both at "low risk" and at higher risk.
Laboratory
In laboratories where diagnostic testing is carried out, biosafety level 4-equivalent containment is required. Laboratory researchers must be properly trained in BSL-4 practices and wear proper PPE.
Putting on protective equipment
 Play media
Play mediaIntroduction
 Play media
Play mediaTrained observer
 Play media
Play mediaRemoving


