Hypoplastic Left Heart Syndrome 1
A number sign (#) is used with this entry because of evidence that hypoplastic left heart syndrome-1 (HLHS1) is caused by mutation in the GJA1 gene (121014) on chromosome 6q22.
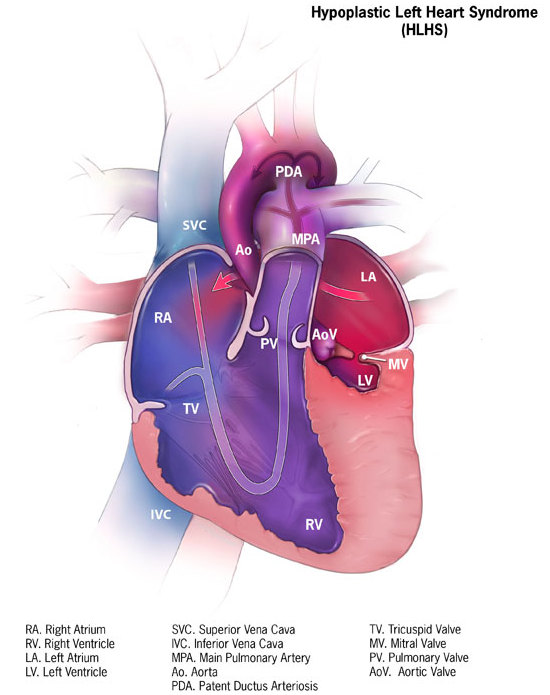
DescriptionHypoplastic left heart syndrome results from defective development of the aorta proximal to the entrance of the ductus arteriosus and hypoplasia of the left ventricle and mitral valve. As a result of the abnormal circulation, the ductus arteriosus and foramen ovale are patent and the right atrium, right ventricle, and pulmonary artery are enlarged (Brekke, 1953).
Genetic Heterogeneity of Hypoplastic Left Heart Syndrome
Hypoplastic left heart syndrome-2 (HLHS2; 614435) is caused by mutation in the NKX2-5 gene (600584) on chromosome 5q35.1.
Somatic mutations in the HAND1 gene (602406) have been identified in tissue samples from patients with HLHS.
Clinical FeaturesBrekke (1953) described 2 brothers, born 2 years apart, who died in the neonatal period. On autopsy, the boys had atresia or hypoplasia of the aortic orifice and hypoplasia of the left ventricle and ascending aorta, patency of the foramen ovale and of the ductus arteriosus, and hypertrophy of the right ventricle and right atrium. A previous pregnancy had resulted in a full-term stillborn infant in which an autopsy was not performed.
Kojima et al. (1969) described hypoplastic left heart syndrome in sibs. Shokeir (1971) described 13 patients in 5 families. Parental consanguinity was present in 3 sibships. In all affected infants, the course of the disease was inexorably progressive and ultimately fatal.
Loffredo et al. (2004) ascertained families of 38 probands with hypoplastic left heart, 46 with coarctation of the aorta (120000), and 22 with d-transposition of the aorta (DTGA; 608808), with the latter group serving as 'disease controls.' Cardiovascular malformations were detected more frequently in first-degree relatives of probands with hypoplastic left heart (19.3%) or coarctation of the aorta (9.4%) than among DTGA families (2.7%). Less than 1% of second-degree relatives were affected in all 3 groups. In third-degree relatives, cardiovascular malformations were detected in 1.8% of families with hypoplastic left heart compared to 1.2% in families with coarctation of the aorta and 0.4% in families with DTGA. The predominant types of malformation seen in relatives were left-sided obstructive lesions. Loffredo et al. (2004) stated that this confirmed the familial aggregation of congenital heart defects among infants with hypoplastic left heart and coarctation of the aorta.
The spectrum of left ventricular outflow tract obstruction (LVOTO) consists of hypoplastic left heart or left ventricle, aortic valve stenosis and bicuspid aortic valve (109730), hypoplastic aortic arch, and coarctation of the aorta (120000). Wessels et al. (2005) described 4 families with presumed autosomal dominant inheritance of LVOTO. In these families, LVOTO showed a wide clinical spectrum, with some members having severe anomalies such as hypoplastic left heart and others having only minor anomalies such as mild aortic valve stenosis. Wessels et al. (2005) concluded that their findings supported the suggestion that all anomalies of the LVOTO spectrum are developmentally related and sometimes can be caused by a single gene defect.
InheritanceHolmes et al. (1974) found a frequency of the hypoplastic left heart syndrome among sibs most consistent with multifactorial inheritance. Brownell and Shokeir (1976) also obtained results most compatible with multifactorial inheritance, the recurrence risk among later-born sibs being about 2%.
McBride et al. (2005) undertook a formal inheritance analysis of LVOTO in 124 families ascertained by an index case with aortic valve stenosis, coarctation of the aorta, or hypoplastic left heart. LVOTO malformations were noted in 30 relatives, along with significant congenital heart defects in 2 others, yielding a total of 32 (7.7%) of 413 relatives. Relative risk for first-degree relatives in this group was 36.9, with a heritability of 0.71 to 0.90. McBride et al. (2005) concluded that their data supported a complex but most likely oligogenic pattern of inheritance.
Molecular GeneticsIn 8 pediatric heart transplant recipients with hypoplastic left heart syndrome, Dasgupta et al. (2001) identified 4 substitutions in the GJA1 gene: 2 missense mutations (see 121014.0001 and 121014.0012) and 2 silent polymorphisms.
Animal ModelLiu et al. (2017) studied 8 mutant mouse lines with hypoplastic left heart syndrome, recovered from chemical mutagenesis of 100,000 fetal mice. Exome sequencing showed 330 coding or splicing mutations, none of which were shared, indicating that HLHS is genetically heterogeneous. In the Ohia mouse line, the authors identified homozygous mutations in 2 different genes, Sap130 (609697) and Pcdha9 (606315); HLHS appeared to arise from synergistic interactions between the Sap130 and Pcdha9 mutations, and HLHS was significantly enriched in double homozygous mutants. Mouse and zebrafish modeling showed that Sap130 mediated left ventricular hypoplasia, whereas Pcdha9 increased penetrance of aortic valve abnormalities. Liu et al. (2017) noted that mutations from the 8 HLHS mouse lines were significantly enriched among multihit genes in human subjects with HLHS compared to CEU controls, and exome sequencing of 68 patients with HLHS identified 15 patients with 17 PCDHA mutations and 1 patient (HLHS-22) who carried a PCDHA mutation and a SAP130 mutation.