Takotsubo Cardiomyopathy
Takotsubo cardiomyopathy or Takotsubo syndrome (TTS), also known as stress cardiomyopathy, is a type of non-ischemic cardiomyopathy in which there is a sudden temporary weakening of the muscular portion of the heart. It usually appears after a significant stressor, either physical or emotional; when caused by the latter, the condition is sometimes called broken heart syndrome. Examples of physical stressors that can cause TTS are sepsis, shock, and pheochromocytoma, and emotional stressors include bereavement, divorce, or the loss of a job. Reviews suggest that of patients diagnosed with the condition, about 70-80% recently experienced a major stressor including 41-50% with a physical stressor and 26-30% with an emotional stressor. TTS can also appear in patients who have not experienced major stressors.
The pathophysiology is not well understood, but a sudden massive surge of catecholamines such as adrenaline and norepinephrine from extreme stress or a tumor secreting these chemicals is thought to play a central role. Excess catecholamines, when released directly by nerves that stimulate cardiac muscle cells, have a toxic effect and can lead to decreased cardiac muscular function or "stunning". Further, this adrenaline surge triggers the arteries to tighten, thereby raising blood pressure and placing more stress on the heart, and may lead to spasm of the coronary arteries that supply blood to the heart muscle. This impairs the arteries from delivering adequate blood flow and oxygen to the heart muscle. Together, these events can lead to congestive heart failure and decrease the heart's output of blood with each squeeze.
Takotsubo cardiomyopathy occurs worldwide. The condition is thought to be responsible for 2% of all acute coronary syndrome cases presenting to hospitals. Although TTS has generally been considered a self-limiting disease, spontaneously resolving over the course of days to weeks, contemporary observations show that the rates of cardiogenic shock and death in TTS are comparable to those of acute coronary syndrome (ACS) patients. These cases of shock and death have been associated with the occurrence of TTS secondary to an enciting physical stressor such as hemorrhage, brain injury sepsis, pulmonary embolism or severe COPD.
It occurs more commonly in postmenopausal women. The name "takotsubo" comes from the Japanese word takotsubo "octopus trap", because the left ventricle of the heart takes on a shape resembling an octopus trap when affected by this condition.
Risk factors
Stress trigger
Although there have been documented cases of TTS without a triggering stressor, it is widely recognized that TTS is preceded by a stressful event. Case series looking at large groups of patients report that some patients develop takotsubo cardiomyopathy after an emotional stress. Some patients have a preceding clinical stressor (such as a brain injury, asthma attack or exacerbation of a chronic illness) and research has indicated that this type of stress may even occur more often than emotionally stressful triggers. Roughly one-third of patients have no preceding stressful event. A 2009 large case series from Europe found that takotsubo cardiomyopathy was slightly more frequent during the winter season. This may be related to two possible/suspected pathophysiological causes: coronary spasms of microvessels, which are more prevalent in cold weather, and viral infections – such as Parvovirus B19 – which occur more frequently during the winter.
Gender
Women, specifically postmenopausal women, are at greatest risk of developing TTS. This has led some researchers to theorize about the possible protective effects of estrogen in preventing TTS.
Genetic risk factors
It is currently being investigated if certain genetic traits associated with catecholamine receptors found on cardiac muscle cells play a role in the development of TTS. There is limited evidence tying TTS directly to a specific genetic expression or mutation, however there is currently a widely held hypothesis supporting the idea of the interaction between environmental factors and the interplay of genetic predisposition leading to the susceptibility to microvascular alterations that contribute to the TTS disease process.
Hormonal dysregulation
Certain endocrine diseases including pheochromocytoma and thyrotoxicosis have been identified as potential risk factors for TTS. The relationship between thyroid function and stress cardiomyopathy is marked by a dual phenotype, where both impending primary hyperthyroidism and a high set point of thyroid homeostasis (encoding type 2 allostatic load) are common phenomena. A multi-centre observation study found normal thyroid function to be the exception rather than the rule in TTS. Especially hyperthyroidism is highly prevalent in takotsubo cardiomyopathy, and it seems to predict a poor prognosis in terms of complications and mortality.
Signs and symptoms
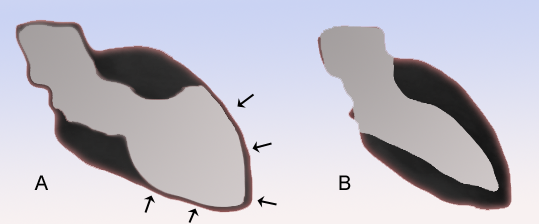
The typical presentation of takotsubo cardiomyopathy is chest pain with or without shortness of breath and associated electrocardiogram (ECG) changes mimicking a myocardial infarction of the anterior wall. During the course of evaluation of the patient, a bulging out of the left ventricular apex with a hypercontractile base of the left ventricle is often noted. It is the hallmark bulging-out of the apex of the heart with preserved function of the base that earned the syndrome its name takotsubo "octopus trap", in Japan, where it was first described.
Stress is the main factor in takotsubo cardiomyopathy, with more than 85% of cases set in motion by either a physically or emotionally stressful event that prefaces the start of symptoms. Examples of emotional stressors include grief from the death of a loved one, fear of public speaking, arguing with a spouse, relationship disagreements, betrayal, and financial problems. Acute asthma, surgery, chemotherapy, and stroke are examples of physical stressors. In a few cases, the stress may be a happy event, such as a wedding, winning a jackpot, a sporting triumph, or a birthday.
Pathophysiology
The cause of takotsubo cardiomyopathy is not fully understood, but several mechanisms have been proposed. It is well documented that elevated catecholamine levels have been implicated in the vast majority of TTS cases. Theories suggest a link between brain activation of stress-related biochemicals and the effects these chemicals have on areas of the heart. More specifically, adrenal stimulation by the sympathetic nervous system has been noted in cases ranging from physical events such as ischemic stroke, to emotional events such as depression or loss of a loved-one. How these increased levels of catecholamines act in the body to produce the changes seen with TTS is not clearly understood. Research supports the widely-held understanding that microvascular dysfunction and coronary vasospasm caused by a rapid influx of catecholamines to cardiac myocytes results in apical stunning and transient cardiomyopathy.
- Microvascular dysfunction/Transient vasospasm: Some of the original researchers of takotsubo suggested that multiple simultaneous spasms of coronary arteries could cause enough loss of blood flow to cause transient stunning of the myocardium. Other researchers have shown that vasospasm is much less common than initially thought. It has been noted that when there are vasospasms, even in multiple arteries, that they do not correlate with the areas of myocardium that are not contracting. However, the idea of coronary artery vasospasm is still believed to contribute to the TTS disease process. The theory of vasospasm is not easily separated from that of microvascular dysfunction and in fact, microvascular dysfunction could explain vasospasticity. Impaired microvascular function is seen in a vast majority, if not all, of patients with TTS and is currently one of the most supported theories. Most of the dysfunction occurs as a result of abnormalities within the endothelial linings of blood vessels supplying the heart. In TTS, these highly sensitive interior linings of the vessels have reduced functionality which create dysregulation of vascular tone and predispose the individual to vasoconstriction. When the increased vasoconstrictor effects of catecholamines are introduced, the result is acute cardiac ischemia.
- Catecholamine-induced myocyte injury: It has been suggested that the response to catecholamines (such as epinephrine and norepinephrine, released in response to stress) leads to heart muscle dysfunction that contributes to takotsubo cardiomyopathy. The effects of this toxicity can be greater in those with a predisposition to anxiety or panic disorders. Delivery of catecholamines (epinephrine, norepinephrine) via circulating blood and through direct delivery from cardiac nerves is increased by the stimulation of stress control centers of the brain. During an emotionally or physically stressful event, brain centers initiate the sympathetic nervous pathways and increase myocardial activity. Excessive catecholamine stimulation has a toxic effect on cardiac muscle cells which creates necrosis of the contractile units of cells similarly seen during acute myocardial infarction. The increased workload of cardiac muscle created by the stimulation of catecholamines, increases the need for more blood and oxygen to these muscles to sustain function. When these demands are unable to be met, the heart is starved of blood and oxygen and begins to die. Included in the cytotoxic sequela of catecholamine toxicity is the molecular transformation of the cardiac myocyte to produce apical stunning.
- Mid-ventricular obstruction, apical stunning: It has been suggested that a mid-ventricular wall thickening with outflow obstruction is important in the pathophysiology. This stunning is largely seen as a protective effect produced by the flood of excess catecholamines into the cardiac muscle cell. Overstimulation of catecholamine receptors create physiological changes in the receptor which has an inverse effect on cardiac cellular function. Termed 'cellular-trafficking', this property of the cardiac muscle cell is actually a molecular transformation of the cell to produce a down-regulation of catecolaminergic sensitivity. This means that in the presence of excess epinephrine, a normal cardiac contraction is inhibited in an effort to reduce energy demands, prevent hyperactivity and spare the integrity of the cell. Further bolstering this idea is the concentration of these kinds of receptors in the heart. Higher concentrations of the receptor effected to produce cardiac stunning are found closer to the apex of the ventricle. This is what creates the classic ballooning effect of the ventricle.
It is likely that there are multiple factors at play that could include some amount of vasospasm and failure of the microvasculature. These factors can overlap and create the complex sequela leading to ischemia and left ventricle contraction abnormality. For instance, estrogen, which confers protection to women by improving blood flow to heart muscle, is one biochemical pathway implicated in the TTS disease process. Once this protective mechanism is reduced through the decreased production of estrogen after menopause, there is thought to be an increase in endothelial dysfunction predisposing an individual to vasoconstriction and cardiac ischemia. An inciting stressful event elicits the release of catecholamines into the blood stream to create increased heart muscle activity and metabolism. This leads to further cardiac microvascular endothelial dysfunction through oxidative stress, alteration of ion-mediated channels, and electrolyte disturbances which ultimately alter myocardial cell membrane permeability and dysfunction. Coupled with direct heart muscle toxicity, this crescendo of factors are implicated in the ballooning and heart failure characteristically seen in TTS.
A 2019 case involved a 60-year-old woman presenting with Takotsubo cardiomyopathy due to over-consumption of wasabi, mistaking it for avocado.
Diagnosis
Several well regarded institutions of medical research have produced clinical criteria useful in diagnosing TTS. One of the first sets of guidelines was initially published in 2004 and again in 2008 by the Mayo Clinic. Other research institutions proposing diagnostic criteria include the Japanese Takotsubo Cardiomyopathy Study Group, Gothenburg University, Johns Hopkins University, the Takotsubo Italian Network and the Heart Failure Associates TTS Taskforce of the European Society of Cardiology. All of the research institutions agree on at least two main criteria needed to accurately diagnose TTS: 1) transient left ventricular wall motion abnormality and 2) the absence of a condition obviously explaining this wall motion abnormality (coronary artery lesion, hypoperfusion, myocarditis, toxicity, etc.). Other commonly acknowledged criteria necessary for diagnosis include characteristic EKG changes and mild to modest elevation in cardiac troponin.
Transient apical ballooning syndrome or takotsubo cardiomyopathy is found in 1.7–2.2% of patients presenting with acute coronary syndrome. While the original case studies reported on individuals in Japan, takotsubo cardiomyopathy has been noted more recently in the United States and Western Europe. It is likely that the syndrome previously went undiagnosed before it was described in detail in the Japanese literature. Evaluation of individuals with takotsubo cardiomyopathy typically includes a coronary angiogram to rule out occlusion of the left anterior descending artery, which will not reveal any significant blockages that would cause the left ventricular dysfunction. Provided that the individual survives their initial presentation, the left ventricular function improves within two months.
The diagnosis of takotsubo cardiomyopathy may be difficult upon presentation. The ECG findings often are confused with those found during an acute anterior wall myocardial infarction. It classically mimics ST-segment elevation myocardial infarction, and is characterised by acute onset of transient ventricular apical wall motion abnormalities (ballooning) accompanied by chest pain, shortness of breath, ST-segment elevation, T-wave inversion or QT-interval prolongation on ECG. Cardiac enzymes are usually negative and are moderate at worst, and cardiac catheterization usually shows absence of significant coronary artery disease.
The diagnosis is made by the pathognomonic wall motion abnormalities, in which the base of the left ventricle is contracting normally or is hyperkinetic while the remainder of the left ventricle is akinetic or dyskinetic. This is accompanied by the lack of significant coronary artery disease that would explain the wall motion abnormalities. Although apical ballooning has been described classically as the angiographic manifestation of takotsubo, it has been shown that left ventricular dysfunction in this syndrome includes not only the classic apical ballooning, but also different angiographic morphologies such as mid-ventricular ballooning and, rarely, local ballooning of other segments.
The ballooning patterns were classified by Shimizu et al. as takotsubo type for apical akinesia and basal hyperkinesia, reverse takotsubo for basal akinesia and apical hyperkinesia, mid-ventricular type for mid-ventricular ballooning accompanied by basal and apical hyperkinesia, and localised type for any other segmental left ventricular ballooning with clinical characteristics of takotsubo-like left ventricular dysfunction.
In short, the main criteria for the diagnosis of takotsubo cardiomyopathy are: the patient must have experienced a stressor before the symptoms began to arise; the patient's ECG reading must show abnormalities from a normal heart; the patient must not show signs of coronary blockage or other common causes of heart troubles; the levels of cardiac enzymes in the heart must be elevated or irregular; and the patient must recover complete contraction and be functioning normally in a short amount of time.
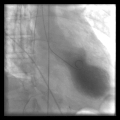
Left ventriculography during systole showing apical ballooning akinesis with basal hyperkinesis in a characteristic takotsubo ventricle
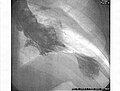
Left ventriculogram during systole displaying the characteristic apical ballooning with apical motionlessness in a patient with takotsubo cardiomyopathy
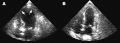
(A) Echocardiogram showing dilatation of the left ventricle in the acute phase (B) Resolution of left ventricular function on repeat echocardiogram six days later
ECG showing sinus tachycardia and non-specific ST and T wave changes from a person with confirmed takotsubo cardiomyopathy
 Play media
Play mediaEchocardiogram showing the effects of the disease
Treatment
The treatment of takotsubo cardiomyopathy is generally supportive in nature, for it is considered a transient disorder. Treatment is dependent on whether patients experience heart failure or acute hypotension and shock. In many individuals, left ventricular function normalizes within two months. Aspirin and other heart drugs also appear to help in the treatment of this disease, even in extreme cases. After the patient has been diagnosed, and myocardial infarction (heart attack) ruled out, the aspirin regimen may be discontinued, and treatment becomes that of supporting the patient. There is currently no internationally agreed protocol for treatment of this condition.
While medical treatments are important to address the acute symptoms of takotsubo cardiomyopathy, further treatment includes lifestyle changes. It is important that the individual stay physically healthy while learning and maintaining methods to manage stress, and to cope with future difficult situations.
Although the symptoms of takotsubo cardiomyopathy usually go away on their own and the condition completely resolves itself within a few weeks, some serious short and long-term complications can happen that must be treated. These most commonly include congestive heart failure and very low blood pressure, and less commonly include blood clotting in the apex of the left ventricle, irregular heart beat, and tearing of the heart wall.
Heart failure
For patients in acute heart failure, ACE inhibitors, angiotensin receptor blockers, and beta blockers, are considered mainstays of heart failure treatment. But use of beta blockers specifically for takotsubo cardiomyopathy is controversial, because they may confer no benefit.
Low blood pressure
For people with cardiogenic shock, medical treatment is based on whether a left ventricular outflow tract (LVOT) obstruction is present. Therefore, early echocardiography is necessary to determine proper management. For those with obstructed LVOTs inotropic agents should not be used, but instead should be managed like patients with hypertrophic cardiomyopathy, (e.g. phenylephrine and fluid resuscitation). For cases in which the LVOT is not obstructed, inotropic therapy (e.g. dobutamine and dopamine) may be used, but with the consideration that takotsubo is caused by excess catecholamines.
Furthermore, mechanical support with an intra-aortic balloon pump (IABP) is well-established as supportive treatment.
Prognosis
Despite the grave initial presentation in some of the patients, most of the patients survive the initial acute event, with a very low rate of in-hospital mortality or complications. Once a patient has recovered from the acute stage of the syndrome, they can expect a favorable outcome and the long-term prognosis is excellent for most. Even when ventricular systolic function is heavily compromised at presentation, it typically improves within the first few days and normalises within the first few months. Although infrequent, recurrence of the syndrome has been reported and seems to be associated with the nature of the trigger. Stress cardiomyopathy is now a well-recognized cause of acute congestive heart failure, lethal abnormal heart rhythms, and rupture of the heart wall.
Epidemiology
Takotsubo cardiomyopathy is rare, affecting between 1.2% and 2.2% of people in Japan and 2% to 3% in Western countries who suffer a myocardial infarction. It also affects far more women than men with 90% of cases being women, most postmenopausal. Scientists believe one reason is that estrogen causes the release of catecholamine and glucocorticoid in response to mental stress. It is not likely for the same recovered patient to experience the syndrome twice, although it has happened in rare cases. The average ages at onset are between 58 and 75 years. Less than 3% of cases occurred in patients under age 50.
History

Rees, et al. wrote in 1967 that the death of a close relative increases the risk of dying within one year by a factor of seven.
Engel wrote about sudden and rapid death during psychological stress in 1971 and itemized 8 causation categories: [1] on the impact of the collapse or death of a close person; [2] during acute grief; [3] on threat of loss of a close person; [4] during mourning or on an anniversary; [5] on loss of status or self-esteem; [6] personal danger or threat of injury; [7] after the danger is over; [8] reunion, triumph, or happy ending. He proposed these events provoke neurovegetative responses, involving both the flight-fight and conservation-withdrawal systems, conducive to lethal cardiac events, particularly in individuals with preexisting cardiovascular disease.
Although the first scientific description of takotsubo cardiomyopathy was not until the 1990s, Cebelin and Hirsch wrote about human stress cardiomyopathy in 1980. The two looked at homicidal assaults that had happened in Cuyahoga County, Ohio, the past 30 years, specifically those with autopsies who had no internal injury, but had died of physical assault. They found that 11 of 15 had myofibrillar degeneration similar to animal stress studies. In the end, they concluded their data supported "the theory of catecholamine mediation of these myocardial changes in man and of the lethal potential of stress through its effect on the heart".
The first studied case of takotsubo cardiomyopathy was in Japan in 1991 by Sato et al. More cases of the syndrome appeared in Japan within the next decade, although western medicine had still not acknowledged it. The syndrome finally occurred in 1997 when Pavin, et al., wrote about two cases of "reversible LV dysfunction precipitated by acute emotional stress." The western world had not heard of such a thing at the time, as it was incredibly rare and often misdiagnosed. The Japanese at last reported about the syndrome to the west in 2001 under the name "transient LV apical ballooning" though at this point the west had already heard of numerous cases. The syndrome reached international audiences through the media in 2005 when the New England Journal of Medicine wrote about the syndrome.



