Wiskott-Aldrich Syndrome
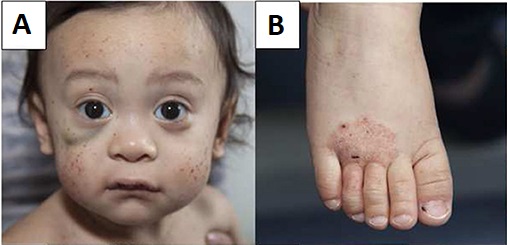
A primary immunodeficiency disease characterized by microthrombocytopenia, eczema, infections and an increased risk for autoimmune manifestations and malignancies.
Epidemiology
The incidence of WAS has been estimated at less than 1 in 100,000 live births. The disease almost exclusively affects males.
Clinical description
WAS usually manifests in infancy but onset may also occur during the neonatal period. In most cases the first clinical features are hemorrhagic manifestations with petechiae, bruising, purpura, epistaxis, oral bleeding, bloody diarrhea and intracranial bleeding. Acute or chronic eczema is the second characteristic finding of WAS. Due to combined immunodeficiency, most patients also have airway, gut or skin infections caused by regular or opportunistic germs. Autoimmune manifestations are seen in approximately 40% of cases and include autoimmune hemolytic anemia, neutropenia, vasculitis, inflammatory bowel disease, renal disease, and arthritis. WAS patients have a higher risk of developing tumors (mainly B-cell lymphomas) at any age.
Etiology
WAS is due to hemizygous mutations in the WAS gene (Xp11.4-p11.21), coding for the Wiskott-Aldrich syndrome protein, exclusively expressed in hematopoietic cells and having a major role in the reorganization of the actin cytoskeleton, signal transduction and apoptosis. Usually, hypomorphic mutations in the WAS gene can lead to an attenuated form of WAS called X-linked thrombocytopenia with normal platelets (XLTT; see this term), that is characterized by mild to moderate thrombocytopenia and eczema and a lower risk of autoimmunity and malignancy, but usually showing no immunodeficiency. Recently, a mutation in the WIPF1 gene (WAS/WASL interacting protein family, member 1; 2q31.2), coding for a protein that stabilizes and prevents the degradation of WASp, was also found in a patient who displayed some features of WAS.
Diagnostic methods
Diagnosis is based on family history, physical examination and laboratory investigations that reveal severe thrombocytopenia with reduced platelet size with a usually normal number of megakaryocytes, as well as altered antibody production (mainly antipolysaccharidic antibodies). Absent or decreased WAS protein levels and genetic testing confirm the diagnosis.
Differential diagnosis
Main differential diagnosis is acute or chronic idiopathic thrombocytopenia (ITP) or platelet alloimmunization in neonates.
Antenatal diagnosis
Prenatal diagnosis is feasible in male fetuses when the causal mutation in the family is known.
Genetic counseling
WAS is an X-linked recessive disease. Carrier women have a 50% risk of transmitting the disease to their male progeny. Some de novo mutations might also occur.
Management and treatment
The only curative treatment to date is hematopoietic stem cell transplantation (HSCT), performed as soon as possible with the best matched HLA donor. In young patients lacking a HLA matched donor, HSCT with a haploidentical donor can lead to a favorable outcome. Gene therapy, still experimental to date, may be a promising approach for patients lacking a suitable donor. Immunoglobulin replacement therapy and oral antibiotics prevent infections. Severe eczema requires treatment with topical or short-term systemic steroids. Treatments that could weaken the immune system (steroids, splenectomy, immunosuppressive agents) should be used with the highest caution by trained medical staff. Agonists of the thrombopoietin receptors (such as romiplostim and eltrombopag) can be used to increase the platelet count in severe refractory thrombocytopenia cases that are awaiting HSCT or gene therapy.
Prognosis
HSCT leads to an 80% survival rate, but when no donor is available, the overall prognosis is poor and life expectancy is reduced, especially when malignancy occurs.