Prader-Willi Syndrome
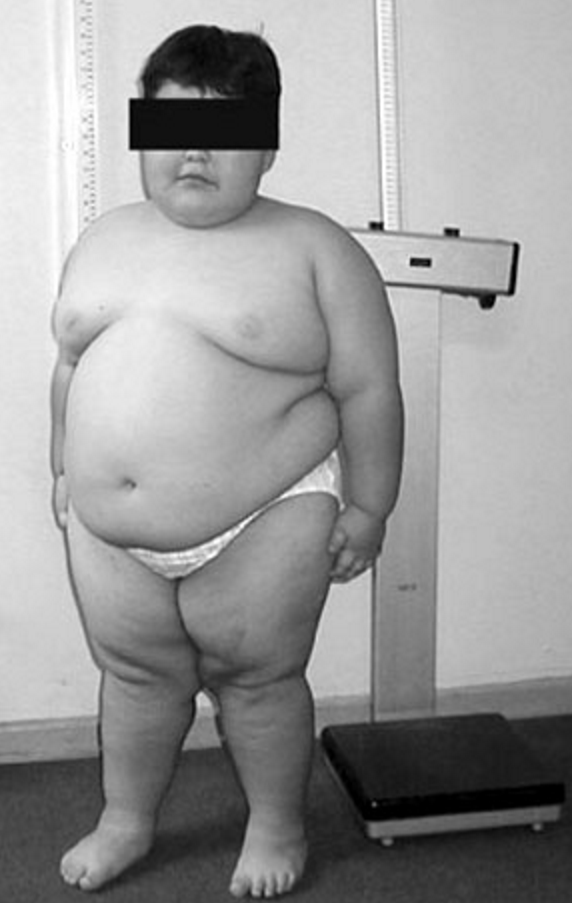
A rare genetic, neurodevelopmental syndrome characterized by hypothalamic-pituitary dysfunction with severe hypotonia and feeding deficits during the neonatal period followed by an excessive weight gain period with hyperphagia with a risk of severe obesity during childhood and adulthood, learning difficulties, deficits of social skills and behavioral problems or severe psychiatric problems.
Epidemiology
Prevalence at birth is estimated at 1/15,000-30,000 worldwide
Clinical description
The severe hypotonia at birth is associated with poor oral and social skills which remain, albeit less clinically evident, throughout life. Characteristic facial features (a narrow forehead, almond-shaped eyes, a thin upper lip and down-turned mouth), as well as very small hands and feet, are frequently observed. After this initial phase, followed by an excessive weight gain without changes in eating, the most striking signs appear: hyperphagia and absence of satiety often leading to severe obesity in affected children as young as three years of age. The situation may deteriorate quickly without strict control of food access. Other associated endocrine abnormalities include short stature due to a growth hormone (GH) deficiency, incomplete pubertal development due to hypogonadism of mixed (central and peripheral) origin, hypothyroidism, premature pubarche and, rarely, corticotropin deficiency. The degree of cognitive dysfunction varies widely but is mild/moderate in most of the individuals. It is associated with learning disabilities, and impaired speech and language development that are aggravated further by psychological and behavioral troubles, impaired social abilities, and control of emotions. Associated comorbidities may include diabetes, sleep-related breathing disorders, gastrointestinal problems, and infections. The Prader-Willi syndrome (PWS) phenotype also occurs in 10% of Fragile X syndrome.
Etiology
The disease is clinically and genetically heterogeneous. Frequently it is caused by either a paternally derived 15q11-q13 deletion, maternal disomy or, very rarely, imprinting defects in the same region.
Diagnostic methods
PWS should be suspected on the presentation of severe neonatal hypotonia, and confirmed by genetic testing which should include methylation analysis, fluorescent in situ hybridization and uniparental disomy testing.
Differential diagnosis
At birth, genetic testing should be used to exclude other causes of hypotonia. If the neonatal phenotype evokes PWS and the genetics are negative, genes for the Prader-Willi-like syndrome (PWS-like) should be searched. In older individuals, the differential diagnosis is of other syndromic obesities such as Bardet-Biedl syndrome, Alström syndrome and, particularly, PWS-like.
Antenatal diagnosis
Diagnosis may be suspected in the last trimester on detection of polyhydramnios, decreased fetal movements and abnormal positions of hand and feet with or without fetal growth restriction. Genetic testing can confirm diagnosis but note that comparative genomic hybridization is not sufficient to exclude PWS.
Genetic counseling
Most cases are sporadic; however, in rare cases dominant transmission may occur with 50% risk where the father carries the imprinting defect.
Management and treatment
Multidisciplinary management should be implemented very early, with particular attention paid to families with psychosocial difficulties. Principally, management is with a strict control of food access and exercise program and, growth hormone (GH) treatment. Associated comorbidities require systematic screening and evaluation. Currently, there are no approved medications to specifically improve the behavioral problems or degree of autonomy obtained. Clinical trials are ongoing for various drugs targeting hyperphagia and behavior.
Prognosis
Obesity is a major factor influencing morbidity and mortality. Early diagnosis, early multidisciplinary care and GH treatment have greatly improved the quality of life of affected children. GH treatment in particular has shown to stabilize body mass index, improve linear growth and adult height and, in children treated before 1 year of age, improve cognitive development. Adolescents benefit from continuing GH treatment Adults who have received GH as children have lower BMI and less comorbidities. Autonomies can be reached but not complete autonomy.